 |
 |
- Search
| Ann Pediatr Endocrinol Metab > Volume 28(4); 2023 > Article |
|
Abstract
Purpose
The correlation between the incidence of type 1 diabetes mellitus (T1DM) and tuberculosis or bacillus Calmette-Gu├®rin (BCG) vaccination rate in individuals aged <15 years was investigated using worldwide data.
Methods
The incidence of T1DM, rate of BCG vaccination, and incidence of tuberculosis were obtained from the Diabetes Atlas 9th edition of the International Diabetes Federation and the Global Health Observatory data repository of the World Health Organization. Gross domestic product (GDP) per capita and population data by country were obtained from the World Bank and United Nations, respectively.
Results
GDP per capita negatively correlated with the incidence of tuberculosis and positively correlated with the incidence of T1DM (coefficient=-0.630 and 0.596, respectively; all P<0.001). The incidence of T1DM and tuberculosis was significantly associated with the Organisation for Economic Cooperation and Development (OECD) status (P<0.001). After adjusting for GDP per capita, regional grouping, and OECD status, the incidence of T1DM negatively correlated with that of tuberculosis (R2 =0.729, P=0.009). However, there was no association between the BCG vaccination rate and incidence of T1DM (P=0.890).
┬Ę Negative correlation was revealed in the incidence of tuberculosis and type 1 diabetes mellitus. Bacillus Calmette-Gu├®rin (BCG) immunization rate did not differ based on the Organisation for Economic Cooperation and Development status and regional grouping. The BCG coverage rate and incidence of type 1 diabetes mellitus showed no correlation.
Type 1 diabetes mellitus (T1DM) is caused by autoimmune destruction of pancreatic beta cells, which synthesize and secrete insulin into the bloodstream [1]. Consequently, patients with T1DM require insulin to survive. Genetic, immunologic, and environmental factors are associated with the pathogenesis of T1DM [2]. Polymorphism of several genes, such as the major histocompatibility complex, protein tyrosine phosphatase, nonreceptor type 22 gene, cytotoxic T-lymphocyte-associated protein 4, and preproinsulin genes, has been reported to affect the development of T1DM [3]. Environmental factors such as viral infections and perinatal or dietary factors may be associated with the development of T1DM [4-6]. Both humoral and cellular immunity contribute to the destruction of beta cells, leading to insulin deficiency [2]. Several B-cell and T-cell targeted immunotherapies were developed to modify to clinical course of T1DM [7].
In the last decade, tumor necrosis factor (TNF) was identified as a potential cytokine that kills autoreactive T cells in subjects with T1DM [8-16]. The bacillus Calmette-Gu├®rin (BCG) vaccine is an attenuated Mycobacterium bovis vaccine that has been used for over 100 years. The vaccine is known to induce TNF by 2 mechanisms, activation of suppressive T regulatory (Treg) cells and death of cytotoxic T lymphocytes (CTLs) that attack pancreatic islet cells [17]. Based on these mechanisms, clinical trials since 2007 have considered BCG therapy for T1DM. A published phase I clinical trial revealed that two doses of the BCG vaccine modified the autoimmune status and lowered glycosylated hemoglobin without causing hypoglycemia in patients with long-term T1DM [17,18]. Currently, a phase II study is recruiting subjects to investigate the sustainability of frequent vaccinations.
Although research has been conducted at the individual level, there are no reports on the association between the epidemiology of T1DM and tuberculosis or BCG vaccinations at the country level. In this study, we investigated the association between the incidence of T1DM and tuberculosis among children and that between incidence of T1DM and BCG immunization coverage among 1-year-old children. These analyses were based on the shared immune metabolism between BCG and tuberculosis [19,20]. This study was performed using data generated by the Diabetes Atlas of the International Diabetes Federation (IDF) and World Health Organization (WHO).
We collected the number of patients with T1DM and tubercu losis younger than 15 years of age. We collected data regarding the incident cases of T1DM in children and adolescents in 2019 from an article in the Diabetes Atlas 9th edition published by the IDF [21]. Countries with estimated data extrapolated from adjacent nations were excluded, and only countries with an existing diabetes registry were included. The countries were classified into seven regions according to the IDF: Africa (AFR), Europe (EUR), Middle East and North Africa (MENA), North America and the Caribbean (NAC), South and Central America (SACA), Southeast Asia (SEA), and Western Pacific (WP). We obtained population numbers by country and age from the United Nations World Population Prospects 2019 [22]. The incidence of T1DM was calculated as patients diagnosed with the disease in 2019 per 100,000 population aged <15 years.
Information on incident tuberculosis cases in individuals aged <15 years [23] and BCG immunization coverage (%) among 1-year-old children [24] were obtained by the WHO Global Health Observatory data repository. Information on the incident cases of tuberculosis in Hong Kong and Taiwan were obtained from the Center for Health Protection under the Department of Health in Hong Kong [25] and the Taiwan Centers for Disease Control [26]. The incidence of tuberculosis was calculated as patients diagnosed with the disease in 2019 per 1,000 population aged <15 years.
Gross domestic product (GDP) per capita by country was compiled by the World Bank for the year 2019 [27]. Membership status of the Organisation for Economic Co-operation and Development (OECD), an intergovernmental organization with 38 high-income developed member countries, was obtained from their website (www.oecd.org).
All statistical analyses were performed using R Statistical Software (ver. 2.14.0; R Foundation for Statistical Computing, Vienna, Austria). Log transformation was applied for the GDP per capita and incidence of T1DM, and square root transformation was applied to determine the incidence of tuberculosis. Following this, the Shapiro-Wilk normality test was performed to assess the normality of the variables. Pearson correlation coefficients were computed for normally distributed data, and Spearman rank correlation coefficients were computed for nonnormally distributed data. In addition, a multiple linear regression model was applied to control the confounding variables and interaction and to analyze the association between the incidence of T1DM and tuberculosis or BCG coverage. A P-value <0.05 indicated statistical significance.
A total of 94 countries had data on the incident cases of T1DM among individuals younger than 15 years in 2019, after excluding the extrapolated data [21]. Of the 94 countries, 91 had data on the incident cases of tuberculosis, while 60 possessed data on BCG immunization coverage rate (Fig. 1). Of these 91 countries, 3 were included in the AFR, 43 in EUR, 12 in MENA, 7 in NAC, 11 in SACA, 4 in SEA, and 11 in WP regions. A total of 37 OECD member countries was enrolled in this study. No OECD member country in the AFR, MENA, and SEA regions was enrolled in the BCG-immunization database. All raw data are listed in Supplementary Table 1.
Among the 91 countries, GDP per capita differed by OECD status and region (P<0.001 and P=0.004, respectively) (Table 1). GDP per capita negatively correlated with the incidence of tuberculosis (coefficient=-0.630, P<0.001) (Fig. 2A) even though it exhibited a positive correlation with the incidence of T1DM (coefficient=0.596, P<0.001) (Fig. 2B). The OECD status was significantly associated with the incidence of T1DM and tuberculosis (all P<0.001). A lower incidence of tuberculosis and a higher incidence of T1DM were observed among individuals in the OECD countries (Table 1).
Among the 91 countries, the incidence of T1DM decreased with an increase in the incidence of tuberculosis (coefficient=-0.490, P<0.001). Adjustment for GDP per capita, region, and OECD status revealed a negative correlation with incidence of tuberculosis and T1DM (coefficient=-5.57, P=0.009) (Fig. 3, Table 2). The EUR and WP regions exhibited a negative correlation between the incidence of tuberculosis and that of T1DM (coefficient=-0.555 and -0.872, all P<0.001) (Supplementary Table 2). Between the OECD and non-OECD member countries in the same region, significantly negative correlations were observed in the non-OECD member countries in the EUR and WP regions (coefficient=-0.749, P=0.002 and coefficient=-0.976, P<0.001) (Supplementary Table 3). As only 3 countries were included in AFR, the region was excluded from statistical analysis among regions. Difference in degree of correlation was noted between the EUR and NAC, NAC and SACA, MENA and WP, and NAC and WP regions (all P<0.05) (Supplementary Table 2).
Of the 60 countries, the BCG immunization rate did not differ based on OECD status or region (P=0.401 and P=0.925, respectively) (Table 1). The BCG vaccination rate and incidence of T1DM did not exhibit linear correlation (coefficient=-0.076, P=0.566). The BCG coverage rate and incidence of T1DM demonstrated no correlation after adjustments for GDP per capita, region, and OECD status (coefficient=0.019, P=0.890) (Fig. 4).
In this study, we identified a negative correlation between the incidence of T1DM and tuberculosis in the pediatric population after adjusting for GDP per capita, region, and OECD status. However, there was no association between BCG immunization and the incidence of T1DM. The incidence of T1DM and tuberculosis exhibited positive and negative associations with the GDP per capita, respectively. The incidence of tuberculosis was lower, while that of T1DM was higher among individuals in OECD countries compared to non-OECD countries.
Tuberculosis is one of the leading causes of death among communicable infections. According to the Global Tuberculosis Report 2020 by the WHO, one-quarter of the world's population is infected with Mycobacterium tuberculosis [28]. The BCG vaccine was introduced in 1921 to prevent the disease and remains in use to date. Although initially developed to prevent tuberculosis, the immune mechanism of BCG leads to the induction of TNF, which expands Treg cells that suppress and kill CTLs that attack pancreatic islet cells. Therefore, TNF inducers demonstrate a protective effect in patients with T1DM. Furthermore, normal immune cells generate energy through oxidative phosphorylation with minimal consumption of sugars or by aerobic glycolysis with a high-glucose transport process. BCG switches the immune metabolism from high reliance on oxidative phosphorylation to aerobic glycolysis [18,19]. In addition, microorganisms utilize aerobic glycolysis. According to the hygiene hypothesis, autoimmune diseases are associated with a lack of interaction between microorganisms and the immune system. Therefore, BCG vaccination suppresses the induction or activation of autoimmunity through several mechanisms. This suggests that BCG therapy could be associated with a favorable effect in patients with T1DM, as reported in phase 1 clinical trials.
In this study, we hypothesized that countries with higher BCG immunization rates of 1-year-old children would exhibit a lower incidence of T1DM. However, no such relationship was found. In previous animal and human studies, administration of BCG at birth did not provide any benefit [29,30]. Furthermore, a single dose of the BCG vaccination was not associated with a reduced incidence of T1DM, and at least 2 doses were needed [18,31]. The BCG vaccine is based on live attenuated mycobacterium strains. The immune response following one dose of the BCG vaccine may not be of similar potency to that against natural infection. The BCG vaccination policies in the BCG atlas [32] showed that, among the enrolled countries, children in only 3 received multiple doses. Therefore, the effects of multiple doses of BCG vaccination could not be evaluated in this study. All three were non-OECD countries with a middle-income group and were located in EUR (Armenia, Bulgaria, and Russian Federation).
However, triggering of TNF release has historically been well-documented after BCG vaccination or tuberculosis infection [32]. Based on the same mechanism, the rate of natural infection caused by Mycobacterium tuberculosis may affect the incidence of T1DM [25,26]. Among the individuals infected with M. tuberculosis, only 5%ŌĆō10% progressed to the disease. In countries where tuberculosis is highly prevalent, individuals are commonly diagnosed with the disease as well as with latent infection. After natural infection or vaccination, autoimmunity is downregulated by the induction of TNF and Treg cells and the suppression of CTLs. Therefore, the incidence of T1DM could be affected at the national level.
The incidence of tuberculosis and T1DM could be influenced by the socioeconomic status. Therefore, the relationship between the 2 diseases was analyzed after adjusting the GDP per capita and based on their OECD status. Likewise, genetic susceptibilities could lead to differences in the incidence of the 2 diseases. According to a systematic review and meta-analyses [34], polymorphism in the (GT)n promoter allele 2 of SLC11A1 gene was significantly associated with the protective effect of T1DM (odds ratio [OR], 0.94; 95% confidence interval [CI], 0.89ŌĆō0.98) and increased incidence of infectious diseases (OR, 1.25; CI, 1.10ŌĆō0.42). The (GT)n allele 3 of the SLC11A1 gene was significantly associated with an increased incidence of T1DM (OR, 1.07; CI, 1.01ŌĆō1.12) and had a protective effect against the occurrence of infectious diseases (OR, 0.83; CI, 0.74ŌĆō0.93). Among the infectious diseases, the (GT)n alleles 2 and 3 were strongly associated with the incidence of tuberculosis (OR, 1.47; CI, =1.30ŌĆō1.66 and OR, 0.75; CI, 0.69ŌĆō0.82, respectively). There was a significant difference based on the ethnicity, especially in AFR.
This study has some limitations. First, given that the measurements of the incidence of tuberculosis and T1DM are only a proxy, caution is necessary when applying the same to the individual level. There is a potential for systematic differences between countries in reporting disease frequency. Data regarding the incidence of both tuberculosis and T1DM can be obtained for a few countries from the WHO and IDF databases, respectively. Second, this is a cross-sectional study and the associations must be analyzed carefully. Last, considering the lack of available data on confounding factors, the results should be interpreted with caution. Despite these limitations, our study intended to further investigate the hypothesis linking tuberculosis infection and the onset of T1DM using exposure data available at the international level. Only data from accredited organizations were used, and extrapolated results were excluded. Furthermore, the immunomechanism of the BCG vaccine against T1DM has already been identified.
In conclusion, there was a negative correlation between the incidence of tuberculosis and T1DM in children and adolescents aged <15 years after adjusting for GDP per capita, region, and OECD status at the country level. However, further studies are necessary to investigate the association at the individual level. Considering the multiple immune mechanisms, BCG vaccination might have a favorable effect on patients with T1DM, and the results of clinical trials can be anticipated.
Supplementary material
Supplementary Tables 1-3 can be found via https://doi.org/10.6065/apem.2244254.127.
Supplementary┬ĀTable┬Ā1.
Incidence rates of tuberculosis and type 1 diabetes, BCG immunization rate among 1-year-old children, GDP per capita and population by country
Supplementary┬ĀTable┬Ā1.
Correlation analysis in each regional group, and comparison of the degree of correlation among regional groups
Supplementary┬ĀTable┬Ā1.
Correlation analysis between OECD countries and non-OECD countries in each regional group
Notes
Funding
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Fig.┬Ā1.
World map showing the enrolled countries in 2019 based on the bacillus Calmette-Gu├®rin immunization rate among 1-year-old children, the incidence of tuberculosis and type 1 diabetes mellitus in children and adolescents aged <15 years (black), countries based on the incidence of tuberculosis and type 1 diabetes mellitus in children and adolescents aged <15 years (gray), and countries without information (white). BCG, bacillus Calmette-Guerin; TB, tuberculosis; T1DM, type 1 diabetes mellitus.
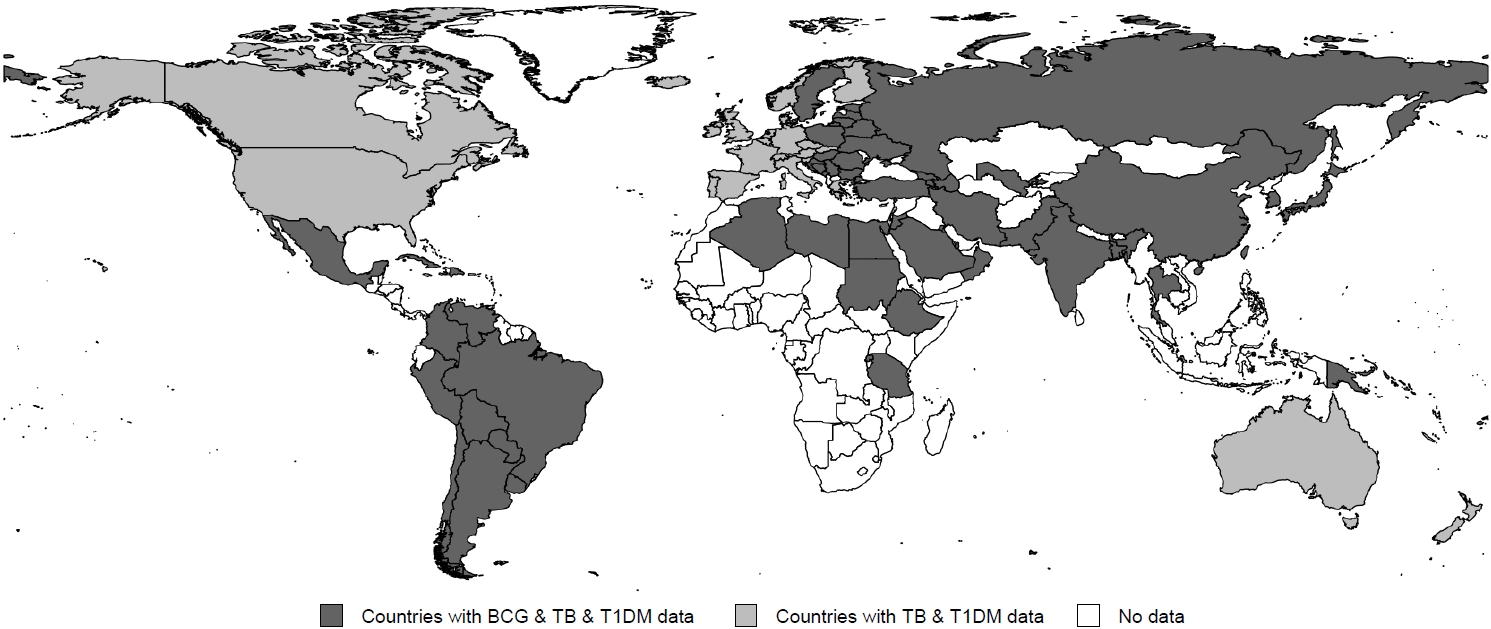
Fig.┬Ā2.
Scatter plot of the associations between the incidence of tuberculosis and gross domestic product per capita (A) and between the incidence of type 1 diabetes mellitus (T1DM) and gross domestic product (GDP) per capita (B). Sqrt, square root transformation.
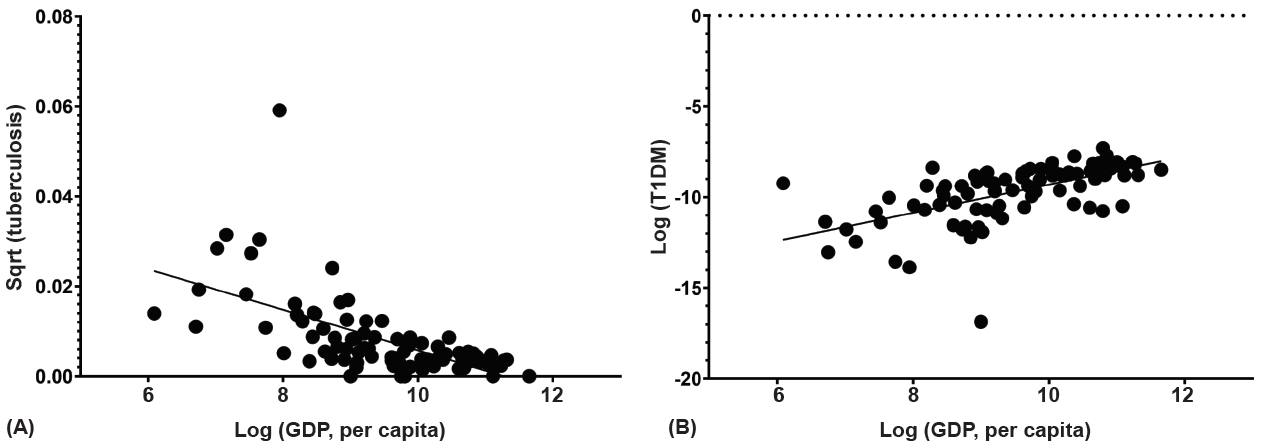
Fig.┬Ā3.
Scatter plot of the association between the incidence of tuberculosis and type 1 diabetes mellitus (T1DM) in children and adolescents aged <15 years. Sqrt, square root transformation.
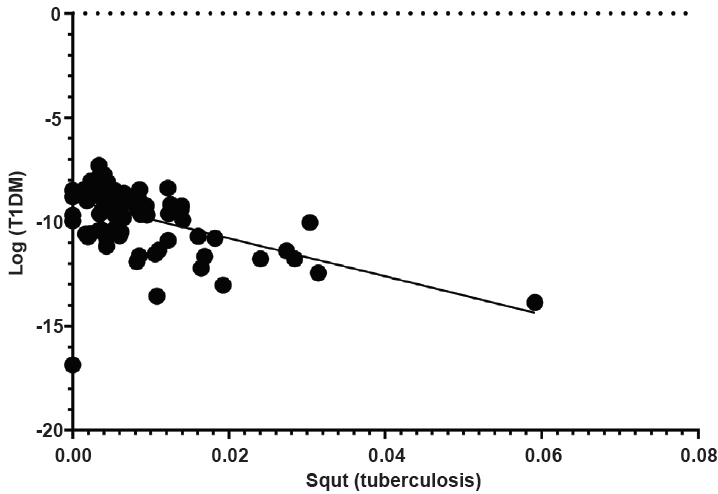
Fig.┬Ā4.
Scatter plot of the association between bacillus Calmette-Gu├®rin (BCG) immunization rate among 1-year-old children and the incidence of type 1 diabetes mellitus (T1DM) .

Table┬Ā1.
Incidence of tuberculosis and T1DM, BCG immunization rate among 1-year-old children, and GDP per capita according to the OECD status and regional groups
T1DM, type 1 diabetes mellitus (T1DM); BCG, bacillus Calmette-Gu├®rin; GDP, gross domestic product; OECD, Organisation for Economic Co-operation and Development; USD, United States dollars; AFR, Africa; EUR, Europe; MENA, Middle East and North Africa; NAC, North America and Caribbean; SACA, South and Central America; SEA, Southeast Asia; WP, Western Pacific.
Table┬Ā2.
Multivariate linear regression analysis of factors influencing in the incidence of type 1 diabetes mellitus
| Variable |
Crude |
Adjusted |
||||
|---|---|---|---|---|---|---|
| Coefficient | SE | P-value | Coefficient | SE | P-value | |
| Incidence of tuberculosis (100,000 people/yr)ŌĆĀ | -2.89 | 0.53 | <0.001 | -5.57 | 2.06 | 0.009 |
| GDP per capita (USD)ŌĆĀ | 0.78 | 0.11 | <0.001 | 3.45 | 3.54 | 0.333 |
| OECD status | ||||||
| ŌĆāNon-OECD | Reference | Reference | ||||
| ŌĆāOECD | 1.58 | 0.29 | <0.001 | -36.28 | 22.73 | 0.115 |
| Regional group | ||||||
| ŌĆāAfrica | Reference | Reference | ||||
| ŌĆāEurope | 3.25 | 0.77 | <0.001 | 22.80 | 24.49 | 0.355 |
| ŌĆāMiddle East and North Africa | 2.41 | 0.84 | 0.005 | 22.56 | 24.35 | 0.357 |
| ŌĆāNorth America and Caribbean | 1.77 | 0.89 | 0.051 | -31.22 | 25.01 | 0.216 |
| ŌĆāSouth and Central America | 1.24 | 0.84 | 0.144 | 9.42 | 24.68 | 0.704 |
| ŌĆāSouth East Asia | 1.29 | 0.99 | 0.196 | 31.55 | 25.25 | 0.216 |
| ŌĆāWestern Pacific | 1.43 | 0.84 | 0.094 | 21.30 | 25.24 | 0.402 |
References
1. Atkinson MA, Maclaren NK. The pathogenesis of insulin-dependent diabetes mellitus. N Engl J Med 1994;331:1428ŌĆō36.


2. Sloboda C, Vedran B, Roberto M. Insulin and type 1 diabetes: immune connections. Eur J Endocrinol 2013;168:R19ŌĆō31.

3. Lee HS, Hwang JS. Genetic aspects of type 1 diabetes. Ann Pediatr Endocrinol Metab 2019;24:143ŌĆō8.




4. Nicola P, Maria G, Sonia B, Susanna E. Type 1 diabetes and viral infections: what is the relationship? J Clin Virol 2017;96:26ŌĆō31.


5. Ingeborg W, Gisela D, Torbj├Črn L. Perinatal risk factors for type 1 diabetes revisited: a population-based register study. Diabetologia 2019;62:1173ŌĆō84.




6. Suvi MV. Dietary factors in the development of type 1 diabetes. Pediatr Diabetes 2016;17 Suppl 22:49ŌĆō55.

7. Smigoc Schweiger D. Recent Advances in Immune-Based Therapies for Type 1 Diabetes. Horm Res Paediatr 2023;96:631ŌĆō45.



8. Faustman D, Davis M. TNF receptor 2 pathway: drug target for autoimmune diseases. Nat Rev Drug Discov 2010;9:482ŌĆō93.



9. Okubo Y, Mera T, Wang L, Faustman DL. Homogeneous expansion of human T regulatory cells via tumor necrosis factor receptor 2. Sci Rep 2013;3:3153.




10. Kodama S, Davis M, Faustman DL. The therapeutic potential of tumor necrosis factor for autoimmune disease: a mechanistically based hypothesis. Cell Mol Life Sci 2005;62:1850ŌĆō62.




11. Satoh J, Seino H, Abo T. Recombinant human tumor necrosis factor a suppresses autoimmune diabetes in nonobese diabetic mice. J Clin Invest 1989;84:1345ŌĆō48.



12. Grewal IS, Grewal KD, Wong FS, Picarella DE, Janeway CA, Flavell RA. Local expression of transgene encoded TNF alpha in islets prevents autoimmune diabetes in non-obese diabetic (NOD) mice by preventing the development of autoreactive islet specific T cells. J Exp Med 1996;184:1963ŌĆō74.




13. Ban L, Zhang J, Wang L, Kuhtreiber W, Burger D, Denise LF. Selective death of autoreactive T cells in human diabetes by TNF or TNF receptor 2 agonism. Proc Natl Acad Sci U S A 2008;105:13644ŌĆō9.



14. Qin HY, Chaturvedi P, Singh B. In vivo apoptosis of diabetogenic T cells in NOD mice by IFN-c/TNF-a. Int Immunol 2004;16:1723ŌĆō32.

15. Christen U, Von Herrath MG. Apoptosis of autoreactive CD8 lymphocytes as a potential mechanism for the abrogation of type 1 diabetes by islet-specific TNF-alpha expression at a time when the autoimmune process is already ongoing. Ann N Y Acad Sci 2002;958:166ŌĆō9.

16. Ryu S, Kodama S, Ryu K, Schoenfeld DA, Faustman DL. Reversal of established autoimmune diabetes by restoration of endogenous beta cell function. J Clin Invest 2001;108:63ŌĆō72.



17. Denise LF, Limei W, Yoshiaki O, Douglas B, Liqin B, Guotong M, et al. Proof-of-Concept, Randomized, Controlled Clinical Trial of bacillus-Calmette-Guerin for Treatment of Long-Term Type 1 Diabetes. PLoS One 2012;7:e41756.



18. Willem MK, Lisa T, Taesoo K, Michael D, Brian N, Sara P, et al. Long-term reduction in hyperglycemia in advanced type 1 diabetes: the value of induced aerobic glycolysis with BCG vaccinations. NPJ Vaccines 2018;3:23.


19. K├╝htreiber WM, Faustman DL. BCG therapy for type 1 diabetes: restoration of balanced immunity and metabolism. Trends Endocrinol Metab 2019;30:80ŌĆō92.


20. Cheng SC, Jessica Q, Robert A C, Kelly M S, Sadia S, Vinod K, et al. mTOR- and HIF-1a-mediated aerobic glycolysis as metabolic basis for trained immunity. Science 2014;345:1250684.


21. Christopher CP, Suvi K, Paraskevi S, Pouya S, Gisela D, Gyula S, et al. Worldwide estimates of incidence, prevalence and mortality of type 1 diabetes in children and adolescents: results from the International Diabetes Federation Diabetes Atlas, 9th ed. Diabetes Res Clin Pract 2019;157:107842.

22. United Nations. World population prospects 2019 [Internet]. New York, United Nations. [cited 2020 Jul 29]. Available from: https://population.un.org/wpp/Download/Standard/Population.
23. World Health Organization. The global health observatory for WHO [Internet]. Geneva (Switzerland), World Health Organization. 2019;[cited 2021 Apr 25]. Available from: https://www.who.int/data/gho/data/themes/tuberculosis/.
24. World Health Organization. The global health observatory for WHO [Internet]. Gene va (Switzerland), World Health Organization. 2019;[cited 2021 Apr 25]. Available from: https://www.who.int/data/gho/data/themes/immunization.
25. Centre for Health Protection HK. Statistics for the HK [Internet]. Hong Kong, Centre for Health Protection. 2019;[cited 2021 Apr 25]. Available from: http://www.chp.gov.hk/en/statistics.
26. Taiwan Centers for Disease Control. Statistics of communicable diseases and surveillance report 2019 [Internet]. Taipei (Taiwan), Taiwan Centers for Disease Control. 2019;[cited 2021 Apr 25]. Available from: https://www.cdc.gov.tw/En/InfectionReport/Info/A1HdZwK8fUzN1IiBKDPkVQ?infoId=v_K-Q-IjnQf9Qj923ZLCqg.
27. The World Bank. GDP per capita (current US$) [Internet]. Washington, D.C, The World Bank. 2019;[cited 2021 Apr 25]. Available from: https://data.worldbank.org/indicator/NY.GDP.PCAP.CD.
28. World Health Organization. Global tuberculosis report. Geneva (Switzerland): World Health Organization Press. 2020.
29. McDevitt H, Munson S, Ettinger R, Wu A. Multiple roles for tumor necrosis factor alpha and lymphotoxin alpha/beta in immunity and autoimmunity. Arthritis Res 2002;4 Suppl 3(Suppl 3):S141ŌĆō52.

30. Huppmann M, Andrea B, Anette-G Z, Ezio B. Neonatal bacille CalmetteŌĆōGuerin vaccination and type 1 diabetes. Diabetes Care 2005;28:1204ŌĆō6.



31. Danise LF. The value of BCG and TNF in autoimmunity. 2nd ed. London: Elsevier/Academic Press. 2018.
32. McGill University. BCG world atlas. 3rd ed [Internet]. Montreal (Canada), McGill University. 2017;[cited 2021 Apr 25]. Available from: http://www.bcgatlas.org; 2017.
- TOOLS
- Related articles in APEM







