 |
 |
- Search
| Ann Pediatr Endocrinol Metab > Volume 28(4); 2023 > Article |
|
Abstract
Purpose
There are no definite guidelines on the optimal dosage of gonadotropin-releasing hormone (GnRH) agonist for treatment of central precocious puberty (CPP). We compared growth outcomes of GnRH agonist at different dosages in girls with idiopathic CPP to assess the optimal dosage.
Methods
This retrospective study included 86 girls with idiopathic CPP who had been treated with GnRH agonist for at least one year and had attained their final adult height. Leuprolide was given as fixed dosage (3.75 mg every 4 weeks in body weight >20 kg, n=72) or weight-based dosage (60ŌĆō85 ╬╝g/kg every 4 weeks, n=14). We compared suppression of advanced puberty and treatment response between the 2 groups.
Results
Peak estradiol and luteinizing hormone and bone age (BA)/chronological age after injection of GnRH agonist were effectively suppressed in both groups. In both groups, the height standard deviation score (SDS) for BA increased after treatment. Final adult height (FAH) (fixed dosage group,160.8┬▒4.1 cm and weight-based dosage group, 161.2┬▒4.4 cm) was significantly higher than the initial predicted adult height (PAH) (155.5┬▒3.3 and 156.1┬▒3.6 cm, respectively) (both P<0.001) and similar to midparental height (159.8┬▒3.3 and 160.6┬▒3.7 cm, respectively). There were no differences in gain in height SDS for BA and gain in height (FAH-PAH at the start) between the 2 groups.
┬Ę This article highlights the optimal dosage of Gonadotropin-releasing hormone (GnRH) agonist for treatment of central precocious puberty. There were no differences in treatment outcome between fixed dosage (3.75 mg/4 wk) and weight-based dosage (60-85 ╬╝g/kg/4 wk) of GnRH agonist.
Precocious puberty is defined as development of secondary sexual characteristics before 8 years of age in girls and 9 years of age in boys [1]. In Republic of Korea, the number of patients diagnosed with precocious puberty has steeply increased. The incidence of central precocious puberty (CPP) in girls younger than 9 years increased 4.6 times from 89.4 to 415.3 per 100,000 girls from 2008 to 2014. In boys younger than 10 years, the incidence of CPP increased 9.2 times from 1.6 to 14.7 per 100,000 boys during the same peroid [2]. CPP is caused by early maturation of the hypothalamic-pituitary-gonadal axis and is pathologic in 40%ŌĆō75% of boys [3] and 10%ŌĆō20% of girls [4,5]. The etiology of CPP varies from idiopathic to tumors or lesions in the central nervous system, genetic abnormalities, or previous exposure to excess sex steroid.
CPP can be treated by gonadotropin-releasing hormone (GnRH) agonists, which desensitize and down-regulate pituitary GnRH receptors. GnRH agonists have been available in monthly (4-week), 3-monthly (12-week), and 6-monthly (24-week) formulations, as well as subcutaneous implants [6,7]. In Korea, monthly and 3-monthly formulations of GnRH agonist are most frequently used [8,9]. Leuprolide acetate is the one of the most commonly used preparations and is generally administered at 1.875 mg every 4 weeks in children weighing less than 20 kg and at 3.75 mg every 4 weeks in those greater than 20 kg [8]. The outcome may vary based on treatment dosage, administration route, patient compliance, and starting age of treatment. It is crucial to use a proper dosage of GnRH agonist because oversuppression of estrogen may eventually decrease bone mineral density and suppress growth [10]. However, the optimal dose of GnRH agonist at the start of treatment is controversial. With leuprolide depot, which is the globally most commonly used GnRH agonist, the treatment dosage in the United States ranges from 7.5 mg to 15 mg every 4 weeks (200ŌĆō300 ╬╝g/kg/4 wk) [11]. In Europe and Asia, leuprolide dosing is standardized at 3.75 mg every 4 weeks (compatible to 80ŌĆō120 ╬╝g/kg/4 wk) in patients with body weight more than 20 kg [12]. However, studies have demonstrated that lower dosages are also effective. Tanaka et el. [13] demonstrated that the minimum dosage of leuprolide acetate to suppress puberty is 30 ╬╝g/kg/4 wk. Some physicians favor a minimum dose of GnRH agonist to suppress puberty but avoid growth suppression. A previous study in Republic of Korea compared the outcomes of 3 dosages of GnRH agonist (70, 90, 110 ╬╝g/kg/mo) in CPP patients and demonstrated that higher dosage is necessary to suppress early puberty, although the final adult height (FAH) remains undetermined [14]. Another previous study comparing the treatment outcomes of 2 dosages of GnRH agonist (80-100 ╬╝g/kg/4 wk or 100ŌĆō110 ╬╝g/kg/4 wk) in Republic of Korean CPP patients demonstrated no significant differences in suppression of puberty and growth outcomes between the 2 groups [15]. However, these studies have limitations in that the dosages of 70ŌĆō110 ╬╝g/kg/4 wk in the range of the standard fixed dosage were used, and data on FAH were not included.
In this study, we aimed to evaluate the growth outcomes in girls with idiopathic CPP who were treated with 1 of 2 doses of GnRH agonist (weight-based lower dosage of 60ŌĆō85 ╬╝g/kg every 4 weeks vs. fixed dosage of 3.75 mg every 4 weeks) and who had attained their FAH. Based on these results, we estimated the most effective dosage of GnRH agonist in the treatment of CPP.
Girls who were diagnosed with idiopathic CPP and treated with leuprolide acetate for at least 1 year and who attained their FAH at the Division of Pediatric Endocrinology, Severance Children's Hospital or Gangnam Severance Hospital from January 2010 to December 2018 were enrolled. Totals of 72 girls in Severance Children's Hospital and 14 girls in Gangnam Severance Hospital were included. Patients were diagnosed with idiopathic CPP under the classic diagnostic criteria [16]: (1) onset of breast development (Tanner stage B2 or above) before 8 years of age in girls, (2) peak luteinizing hormone (LH) level above 5 IU/L in the standard GnRH stimulation test using GnRH (100 ╬╝g, gonadorelin, intravenously), and (3) no evidence of intracranial organic lesions confirmed by magnetic resonance imaging. Patients who had underlying diseases that could affect the onset of puberty, such as hypothyroidism, congenital adrenal hyperplasia, leukemia, and chronic renal disease, and those who had received previous medications affecting growth, such as growth hormone, were excluded from the study.
In this retrospective study, leuprolide acetate was administered at either fixed dosage (3.75 mg/4 wk in patients who weighed more than 20 kg) or weight-based dosage (60ŌĆō85 ╬╝g/kg/4 wk). GnRH agonists were injected subcutaneously every 4 weeks. Treatment was stopped when patient bone age (BA) reached 12 years. All patients were followed every 6 months to confirm effective suppression of puberty by Tanner staging, blood sampling 30 minutes after GnRH agonist injection to assess LH and estradiol levels, and measurement of BA.
We measured height, weight, body mass index (BMI), pubertal status, and BA every 3ŌĆō6 months, as well as midparental height (MPH) and predicted adult height (PAH) at baseline and after treatment. Height was measured 3 times with a Harpenden stadiometer at each visit. BA was estimated by the same pediatric endocrinologists using a left-hand x-ray with the Greulich-Pyle method [17], and PAH was calculated based on the Bayley-Pinneau method [18]. FAH was defined as that when the yearly growth rate was less than 1 cm. MPH was calculated as the average of parental heights minus 6.5 cm. Height SDS was calculated using the growth standard for Korean children and adolescents [19]. Height SDS gain for BA, height gain over the MPH (FAH-MPH), and height gain over the initial PAH (FAH-PAH at the start of treatment) were evaluated to compare the effectiveness of the GnRH agonists. The LH and FSH levels were measured by 2-step immunoenzymatic assay (Beckman Coulter Inc., Brea, CA, USA). The E2 level was measured by competitive binding immunoenzymatic assay (Beckman Coulter Inc.). All assays were validated for their limit of detection and quantification, precision, linearity, and recovery.
In this study, all data were expressed as mean┬▒standard deviation. Independent t-test was performed to compare 2 groups and to compare the outcomes before and after GnRH agonist treatment. A P-value less than 0.05 was considered statistically significant. All statistical analyses were performed using IBM SPSS Statistics ver. 24.0 (IBM Co., Armonk, NY, USA).
Anthropometric and clinical characteristics of the individuals, comprising chronological age (CA), BA, BA over CA (BA/CA), height SDS for CA, height SDS for BA, weight, BMI, MPH, PAH, and yearly growth rate, were compared at the beginning of the study. Overall, there were no pretreatment differences in these clinical factors between the 2 groups (Table 1). CA was 8.2┬▒0.8 and 8.5┬▒0.6 years in the fixed dosage and weight-based dosage group, respectively (P=0.083). BA advancement expressed as BA-CA (1.73┬▒0.72 and 1.06┬▒0.80 years, respectively) and BA/CA (1.22┬▒0.10 and 1.13┬▒0.13) were similar in the 2 groups (P=0.540 and P=0.886). GnRH agonist was administered for 2.9┬▒0.5 years in the fixed dosage group and 3.1┬▒1.1 years in the weight-based dosage group. The actual mean dose of GnRH agonist administered during the study was 130.3┬▒20.5 ╬╝g/kg/4 wk in the fixed dosage group and 75.8┬▒10.2 ╬╝g/kg/4 wk in the weight-based dosage group.
We compared the treatment outcome of the 2 groups at the end of treatment and at their FAH. During GnRH agonist treatment, Tanner stage, peak LH and estradiol after injection of GnRH agonist, and BA advancement were well suppressed in both groups, without significant difference. BA/CA decreased from 1.22┬▒0.10 at the start of treatment to 1.05┬▒0.09 at the end of treatment in the fixed dosage group (P<0.05) and from 1.13┬▒0.13 to 1.05┬▒0.06 in the weight-based dosage group (P<0.05), indicating effective puberty suppression in both groups (Table 2).
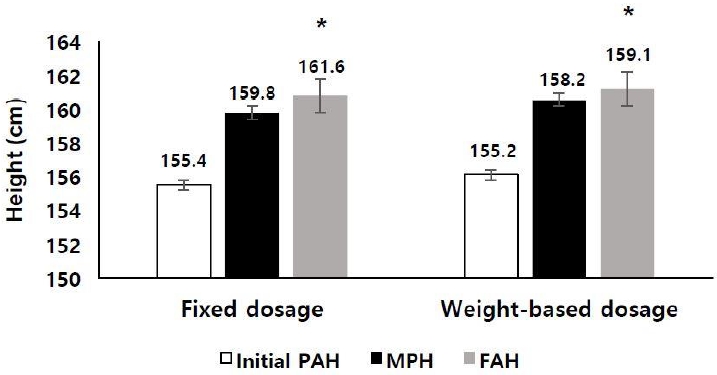
Height SDS for BA increased from -1.22┬▒0.93 at the start of treatment to -1.01┬▒0.76 at the end of treatment in the fixed dosage group (P<0.05) and from -0.53┬▒1.10 to -0.42┬▒0.93 in the weight-based dosage group (P<0.05) (Table 2, Fig. 1). The FAH was 160.8┬▒4.1 cm in the fixed dosage group and 161.2┬▒4.4 cm in the weight-based dosage group (Table 2). PAH increased from 155.5┬▒3.3 cm at the start of treatment to 158.3┬▒3.1 cm at the end of treatment in the fixed dosage group (P<0.05) and from 156.1┬▒3.6 cm to 157.0┬▒3.6 cm in the weight-based dosage group (P<0.05). Height gain over the initial PAH (FAH-PAH at the start of treatment, 5.3┬▒3.4 cm in the fixed dosage group and 5.2┬▒2.9 cm in the weight-based dosage group) (P<0.05) (Fig. 2) and height gain over the MPH (FAH-MPH, 1.0┬▒4.1 cm in the fixed dosage group and 0.7┬▒4.5 cm in the weight-based dosage group) were observed in both groups (Table 3).
FAH was 160.8┬▒4.1 cm in the fixed-dosage group and 161.2┬▒4.4 cm in the weight-based dosage group, with no difference between them (P=0.580). FAH was higher than initial PAH and MPH in both groups (Fig. 2), and there were no differences between the 2 groups (P=0.409 and P=0.862, respectively) in FAH-initial PAH, with a value of 5.3┬▒3.4 cm in the fixed dosage group and 5.2┬▒2.9 cm in the weight-based dosage group (Table 3). Changes in height SDS for BA, FAH-MPH, and FAH-PAH also showed no differences between the 2 groups (Table 3).
We investigated the comparative effectiveness of 2 dosages of leuprolide, the most popular GnRH agonist in Korea, in girls with CPP who had attained their FAH to elucidate the optimal dose for treatment. In our study, there were no significant differences in suppression of puberty and treatment outcomes of FAH, height SDS gain for BA, difference between FAH and MPH, and difference between FAH and initial PAH between fixed dosage (3.75 mg/4 wk, actual mean dose equivalent to 130.3┬▒20.5 ╬╝g/kg/4 wk) and weight-based dosage (60ŌĆō85 ╬╝g/kg/4 wk, actual mean dose equivalent to 75.8┬▒10.2 ╬╝g/kg/4 wk) groups. Administration of a fixed dose of GnRH agonist (3.75 mg in individuals > 20 kg every 4 wk; 1.875 mg in individuals < 20 kg every 4 weeks) is used widely in Europe and Asia [9], as it is convenient to calculate and fractionate the amount of drug required for injection. In contrast, some physicians prefer the weight-based dosage to minimize the growth-suppressing effect by overdose of GnRH agonist. In our study, we demonstrated that the fixed dosage of GnRH agonist can effectively suppress puberty progression without growth suppression. This finding suggests that fixed dosage of GnRH agonist can be used conveniently without precise calculation and fractionation of drug amount for injection. Previous studies have attempted to determine the optimal dose of GnRH agonist for CPP treatment in Korea. Shim et al. investigated 3 dosages of leuprolide acetate (70, 90, and 110 ╬╝g/kg/mo) in 22 girls with early puberty and demonstrated that a higher dose is necessary to suppress early puberty [14]. Jin et al. found no differences in changes of PAH, BA/CA, and height SDS after treatment between 2 dosages of leuprolide acetate (80ŌĆō100 and 100ŌĆō110 ╬╝g/kg/4 wk) in 71 girls with CPP [15]. However, these studies did not investigate the effect of dose of GnRH agonist on the treatment outcomes including FAH, and dosages used in the studies partly overlapped with the range of fixed dosage (3.75 mg every 4 weeks is equivalent to 80ŌĆō120 ╬╝g/kg/4 wk). In our study, we compared the effectiveness of GnRH agonist with distinctly different doses (actual mean dose 130.3┬▒20.5 ╬╝g/kg/4 wk vs. 75.8┬▒10.2 ╬╝g/kg/4 wk) and analyzed growth outcomes based on anthropometric parameters including FAH between groups.
This study has some limitations. First, it was conducted with an insufficient number of participants, which could limit the generalizability of results and indicates the need for caution in their interpretation. In addition, only idiopathic CPP patients were included in this study, although the dose of GnRH agonist required to suppress the hypothalamic-pituitary axis might differ according to cause. Finally, other confounding factors such as nutrition, exercise, and sleep pattern should also be considered when analyzing the outcomes of CPP treatment.
In conclusion, treatment outcomes between fixed dosage and weight-based dosage of GnRH agonist in girls with CPP who had attained their FAH did not differ with effective suppression of puberty. Therefore, a fixed dosage of GnRH agonist can be used more conveniently for CPP treatment without precise calculation and fractionation of the drug for injection. Further studies with a larger number of CPP patients with various causes and different dosages of GnRH agonists should be considered to elucidate the optimal dose for CPP treatment.
Notes
ACKNOWLEDGMENTS
Conceptualization: AK; Data curation: SJK, ML, HIL; Formal analysis: KS; Methodology: JS; Project administration: HWC; Visualization: JS; Writing - original draft: SJ; Writing - review & editing: HK
Fig.┬Ā1.
Changes in height SDS for bone age during treatment in 86 CPP patients treated with GnRH agonist. SDS, standard deviation score; CPP, central precocious puberty; GnRH, gonadotropin-releasing hormone; GnRHa, GnRH agonist. *P<0.05 versus values at the start of GnRHa.
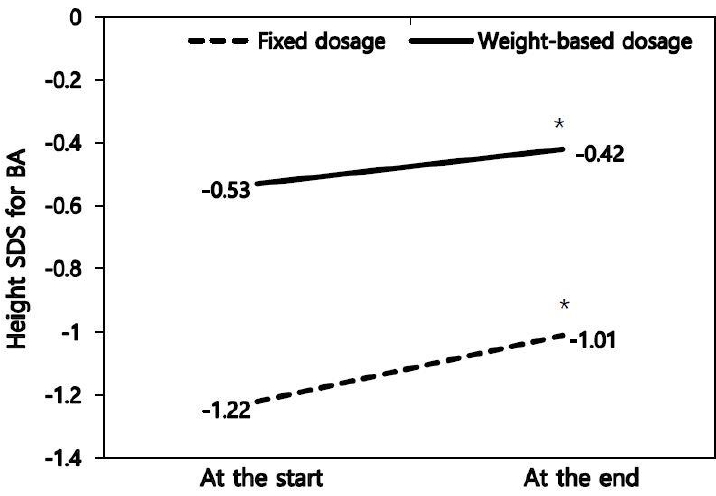
Fig.┬Ā2.
Initial predicted adult height, midparental height, and final adult height in 86 CPP patients treated with GnRH agonist at different dosages. PAH, predicted adult height; MPH, midparental height; FAH, final adult height. *P<0.05 versus values of initial PAH.
Table┬Ā1.
Clinical characteristics of the CPP patients at the start of treatment
Values are presented as mean┬▒standard deviation.
CPP, central precocious puberty; CA, chronological age; BA, bone age; SDS, standard deviation score; BMI, body mass index; MPH, midparental height; PAH, predicted adult height; YGR, yearly growth rate; LH, luteinizing hormone; GnRH, gonadotropin-releasing hormone.
Table┬Ā2.
Comparison of treatment outcomes between dosage groups
| Variable |
Fixed dosage (n=72) |
Weight-based dosage (n=14) |
P-value |
||||||
|---|---|---|---|---|---|---|---|---|---|
| At the start of GnRHa | At the end of GnRHa | At adult height | At the start of GnRHa | At the end of GnRHa | At adult height | At the start of GnRHa | At the end of GnRHa | At adult height | |
| CA (yr) | 8.2┬▒0.8 | 11.2┬▒1.0 | 17.1┬▒0.9 | 8.5┬▒0.6 | 11.3┬▒0.3 | 17.1┬▒0.6 | 0.083 | 0.100 | 0.122 |
| BA (yr) | 9.9┬▒0.8 | 11.8┬▒0.6 | - | 9.6┬▒0.5 | 12.0┬▒0.6 | - | 0.355 | 0.844 | - |
| BA-CA (yr) | 1.73┬▒0.72 | 0.53┬▒1.01 | - | 1.06┬▒0.80 | 0.65┬▒0.71 | - | 0.540 | 0.105 | - |
| BA/CA (yr) | 1.22┬▒0.10 | 1.05┬▒0.09* | - | 1.13┬▒0.13 | 1.05┬▒0.06* | - | 0.886 | 0.079 | - |
| Height (cm) | 131.2┬▒6.2 | 147.5┬▒4.9 | 160.8┬▒4.1 | 133.9┬▒5.1 | 148.9┬▒4.1 | 161.2┬▒4.4 | 0.491 | 0.338 | 0.580 |
| Weight (kg) | 29.5┬▒5.2 | 43.0┬▒7.5 | - | 31.8┬▒5.6.1 | 43.3┬▒7.9 | - | 0.886 | 0.491 | - |
| BMI (kg/m2) | 17.1┬▒1.9 | 19.7┬▒2.6 | - | 17.8┬▒2.8 | 19.7┬▒3.1 | - | 0.286 | 0.890 | - |
| Height SDS for CA | 0.64┬▒1.02 | 0.11┬▒0.95 | 0.12┬▒0.78 | 0.80┬▒0.63 | 0.12┬▒0.66 | 0.18┬▒0.89 | 0.121 | 0.147 | 0.464 |
| Height SDS for BA | -1.22┬▒0.93 | -1.01┬▒0.76* | - | -0.53┬▒1.10 | -0.42┬▒0.93* | - | 0.836 | 0.582 | - |
| PAH (cm) | 155.5┬▒3.3 | 158.3┬▒3.1* | - | 156.1┬▒3.6 | 157.0┬▒3.6* | - | 0.345 | 0.282 | - |
| YGR (cm/yr) | 7.1┬▒3.6 | 4.9┬▒2.0* | - | 6.9┬▒2.8 | 5.9┬▒3.4 | - | 0.626 | 0.087 | - |
| Peak LH (IU/L) | 12.9┬▒11.9 | 1.4┬▒1.1* | - | 11.5┬▒6.8 | 1.2┬▒1.1* | - | 0.340 | 0.734 | - |
| Peak estradiol (pg/mL) | 9.2┬▒4.3 | 8.4┬▒1.0* | - | 7.6┬▒7.7 | 5.6┬▒2.2* | - | 0.082 | 0.089 | - |
| Tanner stage | 2.4┬▒0.5 | 3.0┬▒0.7* | - | 2.0┬▒0 | 2.1┬▒0.3* | - | 0.001 | 0.015 | - |
Table┬Ā3.
Final outcomes of CPP patients treated with 2 different doses of GnRH agonist
References
1. Styne DM, Grumbach MM. Puberty: ontogeny, neuroendocrinology, physiology, and disorders. Kronenberg HM, Melmed S, Polonsky KS, Larsen PRet al., editors. Williams textbook of endocrinology. 12th ed. Philadelphia (PA): Saunders Elsevier. 2011;pp 1054ŌĆō201.
2. Kim YJ, Kwon AR, Jung MK, Kim KE, Suh JH, Chae HW, et al. Incidence and prevalence of central precocious puberty in Korea: an epidemiologic study based on a national database. J Pediatr 2019;208:221ŌĆō8.


3. De Sanctis V, Corrias A, Rizzo V, Bertelloni S, Urso L, Galluzzi F, et al. Etiology of central precocious puberty in males: the results of the Italian Study Group for Physiopathology of Puberty. J Pediatr Endocrinol Metab 2000;13 Suppl 1:687ŌĆō93.

4. Cisternino M, Arrigo T, Pasquino AM, Tinelli C, Antoniazzi F, Beduschi L. Etiology and age incidence of precocious puberty in girls: a multicentric study. J Pediatr Endocrinol Metab 2000;13 Suppl 1:695ŌĆō701.


5. Huynh QTV, Ho BT, Le NQK, Trinh TH, Lam LHT, Nguyen NTK, et al. Pathological brain lesions in girls with central precocious puberty at initial diagnosis in Southern Vietnam. Ann Pediatr Endocrinol Metab 2022;27:105ŌĆō12.




7. Bangalore Krishna K, Fuqua JS, Rogol AD, Klein KO, Popovic J, Houk CP, et al. Use of gonadotropin-releasing hormone analogs in children: update by an International Consortium. Horm Res Paediatr 2019;91:357ŌĆō72.



8. Kim HS. Clinical application of gonadotropin-releasing hormone analogs in children and adolescents. Korean J Pediatr 2010;53:294ŌĆō9.

9. Jeon MJ, Choe JW, Chung HR, Kim JH. Short-term efficacy of 1-month and 3-month gonadotropin-releasing hormone agonist depots in girls with central precocious puberty. Ann Pediatr Endocrinol Metab 2021;26:171ŌĆō7.




10. Vurall─▒ D, Alika┼¤ifo─¤lu A, ─░yig├╝n I, Canoru├¦ D, Ozon A, G├Čn├¦ N, et al. Treatment with depot leuprolide acetate in girls with idiopathic precocious puberty: what parameter should be used in deciding on the initial dose? J Clin Res Pediatr Endocrinol 2020;12:37ŌĆō44.



11. Lee PA, Neely EK, Fuqua J, Yang D, Larsen LM, Mattia-Goldberg C, et al. Efficacy of leuprolide acetate 1-month depot for central precocious puberty (CPP): growth outcomes during a prospective, longitudinal study. Int J Pediatr Endocrinol 2011;2011:7.




12. Carel JC, Eugster EA, Rogol A, Ghizzoni L, Palmert MR; ESPE-LWPES GnRH Analogs Consensus Conference Group, et al. Consensus statement on the use of gonadotropin-releasing hormone analogs in children. Pediatrics 2009;123:e752ŌĆō62.



13. Tanaka T, Hibi I, Kato K, Saito S, Shimizu N, Suwa S, et al. A dose finding study of a super long-acting luteinizing hormone-releasing hormone analog (leuprolide acetate depot, TAP-144-SR) in the treatment of central precocious puberty. The TAP-144-SR CPP Study Group. Endocrinol Jpn 1991;38:369ŌĆō76.

14. Shim KS, Bae CW, Yang YJ. A comparative study of the puberty suppression effect of gonadotropin-releasing hormone agonist in precocious or early puberty girls. Korean J Pediatr 2008;51:634ŌĆō9.

15. Jin HY, Choi JH, Yoo HW. Evaluation of efficacy of GnRH agonist on predicted adult height (PAH) in patients with central precocious puberty using two different dosages. J Korean Soc Pediatr Endocrinol 2010;15:120ŌĆō5.
16. Ab Rahim SN, Omar J, Tuan Ismail TS. Gonadotropin-releasing hormone stimulation test and diagnostic cutoff in precocious puberty: a mini review. Ann Pediatr Endocrinol Metab 2020;25:152ŌĆō5.




17. Greulich WW, Pyle SI. Radiographic atlas of skeletal development of the hand and wrist. 2nd edition. Stanford: Stanford University Press. 1959.
18. Bayley N, Pinneau SR. Tables for predicting adult height from skeletal age: revised for use with the Greulich-Pyle hand standards. J Pediatr 1952;40:423ŌĆō41.


19. Korea Center for Disease Control and Prevention; The Korean Pediatric Society; The Committee for the Development of Growth Standard for Korean Children and Adolescents. 2007 Korean children and adolescents growth standard (commentary for the development of 2007 growth chart). Seoul (Korea): Division of Chronic Disease Surveillance. 2007.
- TOOLS
- Related articles in APEM







