Introduction
Hematopoietic stem cell transplantation (HSCT) has been widely used as crucial treatment for numerous hematological diseases in both adults and children
1). Acute complications such as infections, graft-versus-host disease (GVHD), neurological complications (NCs), and electrolyte imbalance are the major obstacle affecting the outcome of HSCT
2). NCs after HSCT are important causes of morbidity and mortality for transplant recipients and may involve either the central or peripheral nervous systems
3). In patients with acute cerebral nervous system disease, hyponatremia is frequently encountered complications and can either be the result from cerebral salt wasting (CSW) or the syndrome of inappropriate secretion of antidiuretic hormone (SIADH)
4). Hyponatremia is one of the common electrolyte abnormalities in transplant recipients and previous cases reported of various causes of hyponatremia after HSCT, whereas CSW syndrome has been rarely reported
5).
Herein, we present 3 cases of CSW in patients with acute leukemia stratified high risk group who developed severe hyponatremia and polyuria induced by significant renal salt wasting following NC associated with the infection in postengraftment period after HSCT.
Case reports
Case 1
A 16-year-old male with acute myeloid leukemia (AML), who had experienced a relapse after the first bone marrow transplant, developed intermittent fever on day 30 after his second unmatched familial peripheral blood stem cell transplantation (SCT).
Five days later, he presented disorientation and impairment of short term memory. On posttransplant day (PTD) 37, his urine output dramatically increased to 6,690 mL/day (4,430 mL/m2/day), with approximately 2 L of negative fluid balance. The serum sodium level was decreased at 119 mEq/L, followed by generalized tonic-clonic seizure. Considering the possibility of SIADH, fluid restriction was instituted. But severe hyponatremia and significant polyuria persisted and next day and he was consulted to pediatric endocrinologists.
On the past history, vital signs and physical examination at admission was unremarkable. The pretransplantation conditioning regimen comprised busulfan (130 mg/m2), fludarabine (65 mg/m2), and antithymocyte immunoglobulin (ATG, 2.5 mg/kg). Short course methotrexate and cyclosporine were used for the GVHD prophylaxis. Acute GVHD of skin developed at PTD 12 and methylprednisolone (2 mg/kg) was started. Leukocyte engraftment was obtained on PTD 14. Two weeks later, he developed fever. Routine fever studies were performed and urine culture was positive for Klebsiella pneumoniae. Intermittent fever was persisted despite of antibiotics treatment with teicoplanin (10 mg/kg) and meropenem (60 mg/kg). On PTD 35, he developed neurologic symptoms such as disorientation, amnesia and headache. His serum sodium (Na) at this time was 135 mEq/L and the results of lumbar puncture and brain magnetic resonance imaging (MRI) were unremarkable. Two days later, acyclovir (30 mg/kg) was added for continued fever and neurological symptoms.
At the time of consultation (PTD 38), his body weight had decreased by 14% since admission, from 56 kg (25th-50th percentile) to 48.5 kg (5th-10th percentile). Physical examination revealed signs of volume depletion such as weight loss, dry tongue and reduced skin turgor. Central neurologic symptoms with intermittent confusion, disorientation, and short-term memory loss were observed. His serum Na was 114 mEq/L, potassium (K) 3.2 mEq/L, blood urea nitrogen (BUN) 4.6 mg/dL, and osmolality (Osm) 240 mOsm/kg, urine Na 217 mEq/L, and urine Osm 513 mOsm/kg. Thyroid hormone, cortisol, and antidiuretic hormone (ADH) were within normal ranges, while pro-brain natriuretic peptide (pro-BNP) was increased to 534 pg/mL (reference range, <84 pg/mL) and plasma renin activity was suppressed to 0.01 ng/mL/hr (reference range, 0.3-2.9 ng/mL/hr).
The patient was diagnosed as CSW syndrome on the basis of hyponatremia, natriuresis, polyuria with volume depletion, increased pro-BNP level, and absence of response to fluid restriction. Aggressive water and salt replacement was initiated with isotonic and hypertonic saline based on urine output and sodium. Despite adequate fluid replacement, excessive urine output consistently above up to 12,000-14,000 mL (8,000-9,600 mL/m2) with significant urine salt loss of over 200 mEq/L. At this point, pro-BNP levels were significantly elevated to 3,337 pg/mL. To maintain fluid and electrolyte balance, continuous treatment with over 10 L of large volume isotonic fluid and hypertonic saline was required for six day. We then stated fludrocortisone (FC) 0.1 mg per day on PTD 44, but increased urine output was persisted for next 3 days. FC dose was then increased to 0.2 mg per day. Before increased FC, his urine output was 13,320 mL (8,821 mL/m2), serum Na was 130 mEq/L. But 3 days after increasing FC dose, urine output decreased to 5,890 mL (3,875 mL/m2) and serum Na was normalized with this therapy and then hypertonic saline was weaned. On PTD 78, his urine output and serum Na was kept stabilized, FC was weaned. On PTD 83, pro-BNP levels were decreased to 177.2 mL and oral salt tablets were started and 2 weeks later, isotonic fluid was discontinued. On PTD 102, the patient was discharged without neurologic sequelae related with the treatment.
Case 2
A 16-year-old female who was diagnosed with Philadelphia chromosome-positive acute lymphoblastic leukemia presented intermittent fever immediately after transplantation. Fungal pneumonia was developed on PTD 7 and cytomegalovirus (CMV) viremia was diagnosed on PTD 18. The following day, she developed intermittent short-term memory loss, drowsiness and tremor in both hands. Serum Na level was 130 mEq/L and brain MRI was unremarkable. Mild hyponatremia of approximately 130 mEq/L was persisted for next 4 days, urine output dramatically increased to 5,528 mL (4,035 mL/m2), with 1 L of negative fluid balance on PTD 22. Serum Na decreased to 124 mEq/L and she developed seizures. Following conservative fluid treatment using 3% and 0.9% saline for 24 hours, serum Na was normalized. However, the serum Na decreased again to 129 mEq/L on PTD 45, but did not respond to conservative treatment including fluid restriction. Therefore, pediatric hemato-oncologist was consulted on PTD 53.
On the past medical history, The pretransplantation conditioning regimen comprised cyclophosphamide (260 mg/kg), ATG (2.5 mg/kg), methylprednisolone (1 mg/kg), and total body irradiation (total dose, 1,320 cGy/8 fx). Short course methotrexate and cyclosporine were used for the GVHD prophylaxis. Emperical antibiotics with teicoplanin (10 mg/kg) and meropenem (60 mg/kg) were given to her due to fever. On PTD 7, voriconazole (loading dose of 6 mg/kg/dose every 12 hours for 2 doses on day 1, followed by maintenance dose at 4 mg/kg/dose every 12 hours) was initiated due to fungal pneumonia. Leukocyte engraftment was obtained on PTD 12. Six days later, CMV real-time quantitative polymerase chain reaction was positive with 21,000 copies/mL (reference range, <500 copies/mL) and ganciclovir (10 mg/kg) was initiated.
At the time of consultation (PTD 53), her body weight was 36 kg (<3rd percentile), a 15% reduction since admission. Urine output was 2,424 mL (1,924 mL/m
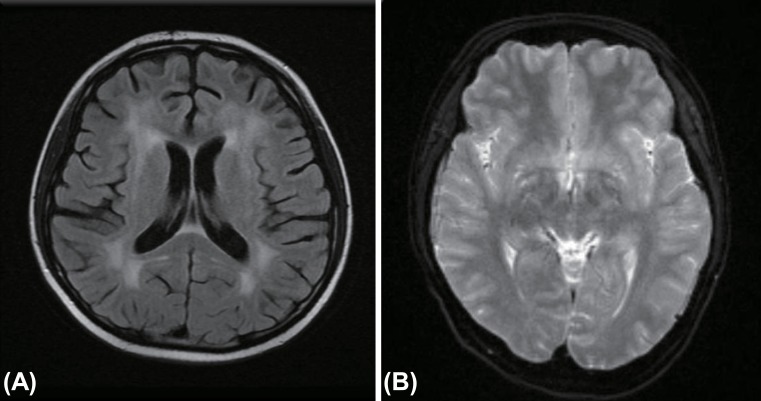
2) with approximately 1.6 L of negative fluid balance. She developed NCs such as disorientation and short-term memory loss. Her serum Na was 126 mEq/L, BUN 21 mg/dL, and Osm 266 mOsm/kg, urine Na 121 mEq/L, and urine Osm 311 mOsm/kg. Thyroid hormones, cortisol and ADH levels were within normal ranges, while BNP was increased to 154 pg/mL (reference range, <100 pg/mL). The clinical pictures and laboratory profiles was highly suggestive of CSW. Her urine volume was replaced by an intravenous fluid with isotonic saline. Two days later, serum Na levels became normal and her vital findings stabilized. Despite stabilized vital signs, appropriate water balance with normal electrolyte levels, her abnormal neurological symptoms such as amnesia, disorientation and tremor persisted until PTD 64. Brain MRI was reperformed and the result showed chemotherapy-related leukoencephalopathy (
Fig. 1A). On PTD 100, the patient was discharged without neurologic sequelae or electrolyte imbalance.
Case 3
A 15-year-old male, who was diagnosed with acute myelocytic leukemia developed fever on PTD 18. The next day, he developed mild headache and tremor in both hands. Three days later, headache was exacerbated and neurologic symptoms include personality changes, decreased activity, drowsiness was developed. Brain MRI revealed prominent pachy-meningeal enhancement and then meningitis was highly suspected (
Fig. 1B). That afternoon, serum Na was rapidly decreased to 128 mEq/L and fluid restriction was instituted. But severe hyponatremia was persisted and pediatric hemato-oncologist was consulted on PTD 22.
The conditioning regimens were comprised of busulfan (130 mg/m2), fludarabine (40 mg/m2), and ATG (2.5 mg/kg). Short course methotrexate and cyclosporine were used for the GVHD prophylaxis. Leukocyte engraftment was obtained on PTD 12. Acute GVHD of skin developed at PTD 13 and methylprednisolone (2 mg/kg) was started. Five days later, he developed fever and emperical antibiotics with teicoplanin (10 mg/kg) and meropenem (60 mg/kg) were initiated.
At the time of consultation (PTD 22), his body weight was 66 kg (75th-90th percentile), a 8% reduction since hospitalization. He had a NCs such as change in personality, slurred and inappropriate speech, truncal ataxia and tremor in both hands. Clinical evaluation revealed signs of volume depletion such as weight loss, decreased skin turgor. His urine output was 4,770 mL (2,650 mL/m
2), with approximately 1 L of negative fluid balance. His serum Na was 127 mEq/L, serum Osm 265 mOsm/kg, urine Na 114 mEq/L, and urine Osm 578 mOsm/kg. While thyroid hormones and ADH levels were within normal ranges, pro-BNP was elevated at 401 pg/mL (reference range, <84 pg/mL). The clinical pictures and laboratory profiles was highly suggestive of CSW. Fluid and salt replacement was instituted with isotonic saline based on urine output and serum Na levels. His serum Na levels began to rise next day. The following day, his serum electrolytes were stabilized and then, clinical condition was improved. Lumbar puncture was performed. The analysis of CSF revealed the following values: red blood cell, 0 cells/mm
3; white blood cell count, 13 cells/mm
3 (4% segmented neutrophil, 40% lymphocytes); protein levels, 82.5 mg/dL; and glucose levels, 59 mg/dL. The findings of an examination of the CSF for tuberculosis and parasites were normal. No pathogens were seen on direct CSF smears, and findings from bacterial and viral cultures remained negative. The diagnosis of CSWS associated with aseptic meningitis was made. We kept continue to use the empirical antibiotics and isotonic fluid treatment. Appropriate positive fluid balance was made by fluid therapy and his serum Na level remained normal levels despite of excessive urine output. His urine output was slowly decreased. The patient was discharged from the hospital with normal electrolyte levels and urine output at day 55 posttransplant (
Table 1).
Discussion
CSWS is defined as a hyponatremia with volume deficits caused by renal loss of sodium during intracranial disease
6). CSWS was first reported by Peters et al. in 1950, when they reported the cases of three patients with various causes of brain disease
7). CSW is mainly reported in patients with central nervous system (CNS) insult, such as CNS hemorrhage, brain injury, tuberculous meningitis, and encephalitis
6)8). However, multiple studies recently reported that it could occur in patients without CNS disease and referred to as renal salt wasting syndrome (RSWS)
9). The salt-wasting syndrome related HSCT are rarely reported. A case of foscarnet administration for human herpesvirus 6 encephalitis induced RSWS on PTD 34 was reported by Najima et al.
10) and two cases of tacrolimus for GVHD prevention induced RSWS on PTD 2 months and 17 months were reported by Yuda et al.
11).
The CNS complications after HSCT are common
12). The clinical manifestations include seizures, headache, altered level of consciousness, involuntary movements, ataxia, speech impairment, and delirium
13)14). The previous studies reported that 70% of the CNS complications occur within the first 100 days after HSCT
3). The causes leading to NC are varies according to the stage of HSCT: from the conditioning, during bone marrow depletion, after engraftment. Infections and related to GVHD is commonly suggested causes during bone marrow reconstitution
13). In our case, all three patients presented intermittent fever and had infections by various causes from Klebsiella in case 1, CMV in case 2 and aseptic meningitis in case 3. Subsequently, NC such as headache, altered consciousness and speech impairment was developed, followed by CSW at one or 2 weeks after engraftment.
The pathophysiology and mechanism of CSWS has not yet been clearly understood. Although there are many theories, this syndrome could be explained with two main hypothesises: interference of sympathetic input to the kidney and release of the circulating natriuretic peptide (NP)
6)15). There are four recognized NP associated with CSWS: atrial NP, BNP, C-type NP, and dendroaspis NP
6). BNP is reported to might be the most probable candidate to mediate CSW
7). BNP is derived from a precursor called pro-BNP, comprised of 108 amino acids, and then cleaved into the biological active BNP (32 amino acids) and an inactive N-terminal fragment (NT-pro BNP, 76 amino acids)
16). The authors measured pro-BNP in cases 1, 3 and BNP in case 2. These NPs have direct natriuretic and diuretic effects by an increase in glomerular filtration rates, a direct effect on the renal medullary tubule, and suppression of the reninaldosterone axis
6). The source of the elevated levels of NP could be either brain-or heart-derived and then would be secreted directly upon brain tissue injury or catecholamine-induced myocardial damage by surges in sympathetic outflow due to acute CNS disease
8). In our cases, the BNP levels were elevated and renin activities were suppressed in all patients.
Hyponatremia is common metabolic complication in patients with cancer or critically ill neurologic disease
5)17). The various causes of hyponatremia were reported such as medications, gastrointestinal loss, infection, and hypothyroidism, adrenal insufficiency, the syndrome of inappropriate antidiuretic hormone secretion (SIADH). Thus, exclusion from other common causes of hyponatremia is crucial for diagnosis of CSWS
18). To date, the reported drug causes of hyponatremia in children who receive chemotherapy or SCT are diuretics, vincristine, vinblastine, cyclophosphamide and cisplatin
19). In our case, among the above drugs, we used diuretics in all patients and cyclophosphamide in case 2. However, the all cases of this report developed hyponatremia and polyuria in the absence of diuretics and hyponatremia related cyclophosphamide was usually occurred within the first 24 hours after administration. And then, both drugs were excluded for cause of hyponatremia. Thyroid function and cortisol levels were within normal ranges in all 3 cases. However, cortisol level could not be measured in case 3.
Differential diagnosis between SIADH and CSWS is clinical challenge, because they both share many laboratory and clinical findings
15). Both diseases are associated with CNS insult and low serum Na, low serum Osm, high urine Na, high urine Osm. The key to distinguishing between CSW and SIADH is accurate assessment of volume status. SIADH is characterized by euvolemic or hypervolemic, whereas CSWS is hypovolemic with symptom of dehydration
6). Assessment of urine output is also important factor to distinguish CSWS from SIADH. SIADH tends to be oliguria, whereas CSWS tends to be polyuria
6). In our case, all patients showed clinical sign of dehydration, polyuria with significant urinary salt loss of over 100 mEq/L.
The mainstay of treatment for CSWS is replacement of water and salt using either isotonic or hypertonic saline, while SIADH is fluid restriction
6)8). The treatments for the two diseases are opposite. Thus, incorrect diagnosis and treatment can be extremely dangerous for patients. Treatment of CSW would be divided into two parts: first, it is necessary to raise natremia to safe levels; second, it is necessary to replace the Na+ pool and volume status of the patient
6).
Our cases showed the successful improvement of hyponatremia with excessive urine output by fluid and salt replacement therapy and that was supported for the diagnosis of CSWS.
Previous cases reported that mineralocorticoid administration is also benefit in CSWS at doses of 0.05 to 1-mg/day cases
20). Fludrocortisone, potent mineralocorticoid was used at doses of 0.2 mg per day in case 1 and showed dramatically improvement of urine output and natriuresis. Fludrocortisone was administrated for 3 weeks, and no side effects were observed.
In summary, we report 3 patients with acute leukemia stratified high risk group who developed severe hyponatremia and marked polyuria following central neurologic complication associated with the infection in postengraftment period after HSCT. The all patients presented hyponatremia, natriuresis, polyuria with clinical sign of hypovolemia and elevated NPs levels were found. All patients improved with fluid and salt replacement and only one patient used FC. Based on the experience, assessment of accurate volume status and laboratory test, such as serum and urine Na levels, ADH and NP levels would be helpful for accurate diagnosis for CSWS. Monitoring of the changes of urine amount, electrolyte and NP levels may offer a benefit to the treatment.
We suggest that CSWS should be considered when a HSCT recipients presented severe hyponatremia, natriuresis and polyuria with clinical signs of dehydration after NC, especially in acute leukemia stratified high-risk group.