 |
 |
- Search
| Ann Pediatr Endocrinol Metab > Volume 18(4); 2013 > Article |
Abstract
Purpose
The rapid increase in the incidence of precocious puberty in Korea has clinical and social significance. Gonadotropin-releasing hormone (GnRH) stimulation test is required to diagnose central precocious puberty (CPP), however this test is expensive and time-consuming. This study aimed to identify factors that can predict a positive response to the GnRH stimulation test.
Methods
Clinical and laboratory parameters, including basal serum luteinizing hormone (LH), follicle-stimulating hormone (FSH), and estradiol (E2), were measured in 540 girls with clinical signs of CPP.
Results
Two hundred twenty-nine of 540 girls with suspected CPP had a peak serum LH level higher than 5 IU/L (the CPP group). The CPP group had advanced bone age (P<0.001), accelerated yearly growth rate (P<0.001), increased basal levels of LH (P=0.02), FSH (P<0.001), E2 (P=0.001), and insulin-like growth factor-I levels (P<0.001) compared to the non-CPP group. In contrast, body weight (P<0.001) and body mass index (P<0.001) were lower in the CPP group. Although basal LH was significantly elevated in the CPP group compared to the non-CPP group, there was considerable overlap between the 2 groups. Cutoff values of basal LH (0.22 IU/L) detected CPP with 87.8% sensitivity and 20.9% specificity.
Precocious puberty is defined as the development of secondary sexual characteristics before 8 years of age in girls and 9 years of age in boys. Most cases result from inappropriate early activation of the hypothalamic-pituitary-gonadal (HPG) axis, termed central precocious puberty (CPP)1). Most cases of CPP are idiopathic CPP, which is diagnosed after excluding identifiable causes such as brain lesions, tumors, hydrocephalus, or head trauma2). The incidence of sexual precocity is increasing rapidly in Korea, and has recently been recognized as an emerging issue3). CPP can compromise height potential or result in psychosocial disturbances for those affected4,5). It has been reported that early-maturing girls display more problem behaviors because of the gap between their and their peers' physical appearance5). Therefore, careful evaluation of pubertal signs, followed by early diagnosis and proper treatment, are important for the girls with suspected sexual precocity6). The diagnosis of CPP can be made by documentation of early activation of HPG axis in a child with progressive sexual development, accelerated growth rate, and advanced bone maturation. Currently, the gonadotropin-releasing hormone (GnRH) stimulation test is the standard method used to verify the activation of HPG axis7). However, the GnRH stimulation test requires multiple blood sampling over long time periods, and sometimes, it needs to be repeated multiple times before a diagnosis of CPP can be rendered. Therefore, we investigated the clinical and laboratory factors that predict positive results on the GnRH stimulation test because knowledge of these factors may help determine the patient selection and timing for the test.
This study aimed to identify factors that can predict a positive response on GnRH stimulation test in girls with suspected precocious puberty. Clinical and laboratory characteristics of the patients with precocious puberty were analyzed at the time of GnRH stimulation test. In addition, the predictive values of basal LH, follicle-stimulating hormone (FSH), and estradiol (E2) levels for diagnosis of CPP were examined.
This study enrolled 540 girls who visited the Pediatric Endocrinology Clinic at Severance Children's Hospital for suspected CPP from March 2007 to June 2011. All subjects had onset of breast development before 8 years of age, accelerated growth rate, and bone age (BA) advancement at least 1 year compared to chronological age (CA). GnRH stimulation test was performed before 9 years of age. Among all subjects, 124 cases performed second GnRH stimulation test at 6-month interval, and only the data from last test were included. All subjects were evaluated for the following clinical and biochemical characteristics, such as pubertal stage, BA, CA, height, height standard deviation score (SDS), weight, weight SDS, body mass index (BMI, kg/m2), BMI SDS, yearly growth rate, basal LH, basal FSH, basal E2, insulin-like growth factor-I (IGF-I), IGF-I SDS, IGF binding protein-3 (IGFBP-3), and IGFBP-3 SDS. In 124 subjects followed up for 6 months for second GnRH stimulation test, ratio of increase of BA to increase of CA (delta BA/delta CA) was investigated. Breast development was determined according to the Marshall and Tanner staging system8). BA was determined on the basis of the Greulich and Pyle method9), by performing radiography of the left hand and left wrist at the GnRH stimulation test. The height, weight, BMI, height SDS, weight SDS, and BMI SDS were calculated using the Korean children and adolescents' growth standard10). IGF-I SDS and IGFBP-3 SDS were converted from reference values to account for CA11). During the GnRH stimulation test, the level of LH and FSH were checked just before GnRH administration, and then again at 30, 60, 90, and 120 minutes after injection of 100 g of GnRH. Serum levels of basal LH and FSH were determined using the sequential 2-step immunoenzymatic assay (Access hLH, FSH Reagent Pack, Beckman Coulter Inc., Brea, CA, USA) with an intra-assay coefficient of variation (CV) of 3.5-5.4%, inter-assay CV of 4.3-6.4%, and a lower limit of detection of 0.2 IU/L for both gonadotropins. Serum E2 levels were measured using the radioimmunoassay (Coat-A-Count Estradiol, Siemens, Erlangen, Germany) with an intra-assay CV of 4.0-7.0%, inter-assay CV of 4.2-8.1%, and a lower limit of detection of 8 pg/mL. Serum IGF-I and IGFBP-3 levels were measured by chemiluminescence immunoassay (Liaison IGF-I Reagent, Liaison, Atlanta, GA, USA). CPP was diagnosed when the peak LH level was Ōēź5 IU/L on GnRH stimulation test. Other endocrine disorders in subjects or the presence of intracranial lesions detected by sella magnetic resonance imaging (MRI) were excluded. This study was approved by the Institutional Review Board of Severance Hospital (No. 4-2013-0377).
Statistical analyses were performed using IBM SPSS ver. 20.0 (IBM Co., Armonk, NY, USA). Student t-test was used to compare the parameters between the groups with positive and negative results on the GnRH stimulation test. The receiver operating characteristic (ROC) curve analysis was used to assess the sensitivity and specificity of the variable factors. P<0.05 were considered statistically significant.
Of the 540 girls enrolled in this study, 229 had a positive result on the GnRH stimulation test and were therefore diagnosed with CPP, whereas the remaining 311 girls had a negative result. Height SDS was similar in both the CPP and non-CPP groups (0.92┬▒0.89 vs. 0.84┬▒0.95, P=0.34). Weight SDS (0.53┬▒0.77 vs. 0.79┬▒0.89, P<0.001) and BMI SDS (0.09┬▒0.92 vs. 0.54┬▒1.04, P<0.001) were lower in the CPP group than in the non-CPP group. Yearly growth rate was higher in the CPP group than in the non-CPP group (8.88┬▒2.91 cm/yr vs. 6.36┬▒2.20 cm/yr, P<0.001). BA advancement was higher in the CPP group than in the non-CPP group (10.11┬▒0.64 yr vs. 9.74┬▒0.97 yr, P<0.001), but the difference between the BA and the CA (BA-CA) was not significant between the 2 groups (1.81┬▒0.63 years vs. 1.78┬▒0.72 years, P=0.59) (Table 1). Ratio of increase of BA to increase of CA (delta BA/delta CA) was not different between the 2 groups (1.16┬▒0.94 vs. 0.99┬▒0.85, P=0.43) (Table 1).
Basal LH levels (0.93┬▒1.07 IU/L vs. 0.75┬▒0.61 IU/L, P=0.02) and basal FSH levels (3.53┬▒1.84 IU/L vs. 2.06┬▒1.06 IU/L, P<0.001) were higher in the CPP group than in the non-CPP group (Table 1). However, there was a considerable overlap between the 2 groups. Notably, only 45 of the 311 non-CPP girls (14.5%) had basal LH levels less than 0.2 IU/L (the minimum detectable level) (Fig. 1). In contrast, 20 of the 229 girls with CPP (8.7%) had basal LH levels less than 0.2 IU/L (Fig. 1). The basal LH/FSH ratio was lower in the CPP group than in the non-CPP group (0.36┬▒0.63 vs. 0.47┬▒0.58, P=0.03). Basal E2 level (10.59┬▒4.44 pg/mL vs. 9.09┬▒2.93 pg/mL, P=0.001) and IGF-I SDS (0.30┬▒0.94 vs. -0.06┬▒0.80, P<0.001) were higher in the CPP group than in the non-CPP group, whereas IGFBP-3 SDS was similar between the 2 groups (-0.50┬▒0.98 vs. -0.51┬▒0.89, P=0.88) (Table 1).
The clinical and biochemical parameters considered to be related to the results of GnRH stimulation testing (Table 1) were adjusted using binary logistic regression analysis. After regression analysis, basal FSH (odds ratio [OR], 2.18; 95% confidence interval [CI], 1.749-2.708; P<0.001), yearly growth rate (OR, 1.49; 95% CI: 1.3431-1.659; P<0.001), IGF-I SDS (OR, 1.32; 95% CI, 1.002-1.737; P=0.048), and BMI SDS (OR, 0.58; 95% CI, 0.372-0.899; P=0.015) were significantly related to a positive response on GnRH stimulation, whereas basal LH, basal E2, and weight SDS were not (Table 2).
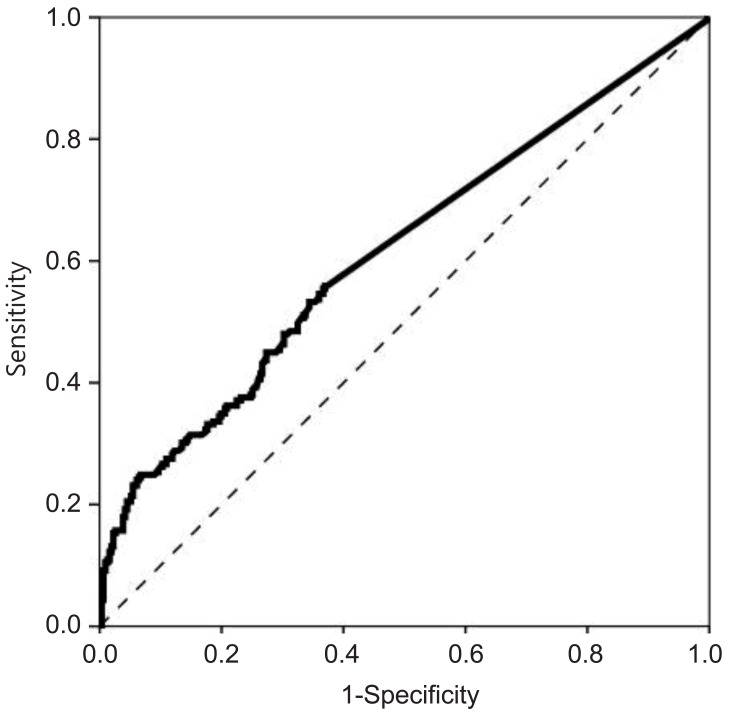
The optimal cutoff values for basal LH, FSH, and E2 to discriminate between girls with and without CPP were determined using ROC curves. The optimal cutoff value of basal LH was 0.22 IU/L, with a sensitivity of 87.8% and a specificity of 20.9% (area under the ROC curve, 0.529) (Fig. 2). The cutoff value of basal FSH was 2.30 IU/L, with a sensitivity of 76.4% and a specificity of 65.9% (area under the ROC curve, 0.78) (Fig. 3). The cutoff value of basal E2 was 8.30 pg/mL, with a sensitivity of 53.3% and a specificity of 65.6% (area under the ROC curve, 0.615) (Fig. 4).
CPP is the most common form of precocious puberty and often results in adverse effects on both physical growth and psychological maturation. Therefore, an early diagnosis followed by immediate treatment is essential to improve both the physical and mental well-being of girls with CPP. Currently, GnRH-stimulated LH levels are the gold standard diagnostic biomarker of CPP. However, GnRH stimulation testing is expensive, time-consuming, and often requires repeated tests before a definitive diagnosis can be made. To avoid unnecessary testing and patient discomfort and to reduce costs, several previous studies have attempted to identify clinical and laboratory biomarkers that may be used to screen patients with suspected CPP in order to guide the proper timing of GnRH testing.
These studies reported that basal LH levels could alone diagnose CPP accurately. LH level could be a useful parameter because testing of LH level is simple, entails minimal patient discomfort, and is quicker to perform compared to the GnRH stimulation test. Neely et al.12) have reported that in 49 girls with clinical signs suggestive of CPP, spontaneous LH levels correlated strongly with peak stimulated LH levels. Therefore, they suggested that LH level could be a useful screening tool for CPP. They demonstrated that spontaneous LH levels in excess of 0.1 IU/L predicted CPP with 94% sensitivity and 88% specificity. Moreover, random LH levels in excess of 0.3 IU/L had 100% specificity for CPP. Similarly, Houk et al.13) investigated basal LH levels in 55 girls with suspected CPP using 2 chemiluminescent assays (Delfia, Wallac Oy, Turku, Finland; Architect, Abbott Park, IL, USA), and assessed the utility of using a single sample to diagnose CPP. They reported that a basal LH cutoff value of 0.83 U/L in the Delfia assay had a sensitivity of 93% and a specificity of 100%, whereas a cutoff value of 1.05 U/L in the Architect assay had a sensitivity of 100% and a specificity of 100%. They therefore suggested that a single basal LH measurement is adequate to document a pubertal state in most-but not all-girls with CPP. Additionally, Pasternak et al.14) reported that a basal LH cutoff value of 0.1 IU/L had a specificity of 94.7% and a sensitivity of 64.4%, suggesting that a single basal LH measurement is adequate to confirm, but not to refute, the presence of CPP.
In our study, basal LH was significantly higher in the CPP group than in the non-CPP group. However, only 14.5% of non-CPP patients had basal LH levels less than 0.2 IU/L (the minimum detectable level), whereas 8.7% of CPP patients did, suggesting there was a considerable overlap between the 2 groups. Moreover, a basal LH cutoff value of 0.22 IU/L determined from ROC curves had a sensitivity of 87.8% and a specificity of 20.9%, suggesting that basal LH levels do not have a sufficiently high sensitivity or specificity to accurately diagnose CPP. Our results also demonstrated that basal FSH, basal E2, and the basal LH/FSH ratio do not have predictive value for the diagnosis of CPP. A basal FSH cutoff value of 2.30 IU/L and a basal E2 cutoff value of 8.30 pg/mL had sensitivities of 76.4% and 53.3% and specificities of 65.9% and 65.6%, respectively. The basal LH/FSH ratio is considered to be elevated in girls with CPP15). In contrast, our results showed that the basal LH/FSH ratio was significantly higher in the non-CPP group than in the CPP group, and an appropriate cutoff value for basal LH/FSH ratio that had both high sensitivity and high specificity could not be determined. Thus, a single laboratory parameter that can predict a positive result on GnRH stimulation with both high sensitivity and high specificity could not be determined in our study. This contradiction to the findings of previous studies may relate to the difference in subject numbers between studies. A previous study using a larger cohort16) also reported that a single basal LH measurement was not adequate to confirm CPP. Another large cohort study composed of 803 girls reported that a basal LH cutoff value of 1.1 IU/L had a sensitivity of 69.1% and a specificity of 50.5%17). These results from large cohort studies, including ours, suggest that a single basal LH measurement is not specific or sensitive enough to diagnose CPP and should therefore not be used as a screening test alone.
Furthermore, we assessed many clinical and laboratory parameters that may predict a positive result by GnRH stimulation test in this study. We found that BA, yearly growth rate, serum IGF-I levels, and IGF-I SDS were higher in girls with CPP than in those without CPP, whereas weight SDS, BMI, and BMI SDS were lower in girls with CPP. In addition, binary logistic regression analysis showed that accelerated yearly growth rate, high IGF-I SDS, and low BMI SDS were significantly correlated with a positive result on the GnRH stimulation test. The most reliable clinical measurement was accelerated yearly growth rate, in accordance with the findings of a previous study in a large cohort16). However, use of yearly growth rate has limitations because at least 3 months are required to assess the growth velocity. Furthermore, we could not determine a proper cutoff value to diagnose CPP using the yearly growth rate. BA advancement and ratio of increase of BA to increase of CA also could not be used to diagnose CPP in our study because these parameters were observed in both groups to the same degree. Several studies have reported that serum IGF-I levels were elevated in girls with CPP, suggesting that serum IGF-I levels are related to CPP16,18). In our study, we analyzed IGF-I SDS to adjust the value according to sex and age using reference values from Korean children and adolescents11). Binary logistic regression analysis showed that serum IGF-I SDS was significantly correlated with CPP, but its predictive value was weak. Previous studies have reported that obesity is associated with earlier pubertal maturation and therefore might be one of the reasons for the general trend towards earlier pubertal onset19,20). However, obesity is also reported to be associated with early pubertal maturation in girls but delayed pubertal onset in boys21), so the exact relationship between obesity and precocious puberty is not fully determined. In our study, low BMI SDS was significantly correlated with a positive result on the GnRH stimulation test.
In conclusion, the present study demonstrated that girls with CPP had an accelerated growth rate, advanced BA, and increased basal gonadotropin and IGF-1 levels. We were unable to identify one individual biochemical parameter that could accurately predict a positive result on the GnRH stimulation test. Therefore, we suggest that multiple factors, such as clinical findings and basal gonadotropin levels, should be considered when evaluating sexual precocity and determining the proper timing for GnRH stimulation testing.
References
1. Merke DP, Cutler GB Jr. Evaluation and management of precocious puberty. Arch Dis Child 1996;75:269ŌĆō271. PMID: 8984908.



2. Cisternino M, Arrigo T, Pasquino AM, Tinelli C, Antoniazzi F, Beduschi L, et al. Etiology and age incidence of precocious puberty in girls: a multicentric study. J Pediatr Endocrinol Metab 2000;13(Suppl 1):695ŌĆō701. PMID: 10969911.



3. Hur K, Park M. Five year national trend of precocious puberty in Korean children, 2004-2008 In: Program and Abstracts, the 58th Annual Fall Meeting of the Korean Pediatric Society; 2009 Oct 23-24; Seoul, Korea. Seoul, Korean Pediatric Society. 2009;pp 179.
4. Brauner R, Adan L, Malandry F, Zantleifer D. Adult height in girls with idiopathic true precocious puberty. J Clin Endocrinol Metab 1994;79:415ŌĆō420. PMID: 8045957.


5. Tremblay L, Frigon JY. Precocious puberty in adolescent girls: a biomarker of later psychosocial adjustment problems. Child Psychiatry Hum Dev 2005;36:73ŌĆō94. PMID: 16049645.


6. Berbero─¤lu M. Precocious puberty and normal variant puberty: definition, etiology, diagnosis and current management. J Clin Res Pediatr Endocrinol 2009;1:164ŌĆō174. PMID: 21274291.


7. Lee PA. Laboratory monitoring of children with precocious puberty. Arch Pediatr Adolesc Med 1994;148:369ŌĆō376. PMID: 8148936.


8. Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child 1969;44:291ŌĆō303. PMID: 5785179.



9. Greulich WW, Pyle SI. Radiographic atlas of skeletal development of the hand and wrist. 2nd ed. Stranford (CA): Stranford University Press. 1959.
10. Korea Centers for Disease Control and Prevention, Division of Chronic Disease Surveillance, Committee for the Development of Growth Standard for Korean Children and Adolescents. Korean Pediatric Society, Committee for School Health and Public Health Statistics. 2007 Korean children and adolescents growth standard (commentary for the development of 2007 growth chart). Cheongwon: Korea Centers for Disease Control and Prevention, Division of Chronic Disease Surveillance. 2007.
11. Hyun SE, Lee BC, Suh BK, Chung SC, Ko CW, Kim HS, et al. Reference values for serum levels of insulin-like growth factor-I and insulin-like growth factor binding protein-3 in Korean children and adolescents. Clin Biochem 2012;45:16ŌĆō21. PMID: 22032863.


12. Neely EK, Wilson DM, Lee PA, Stene M, Hintz RL. Spontaneous serum gonadotropin concentrations in the evaluation of precocious puberty. J Pediatr 1995;127:47ŌĆō52. PMID: 7608810.


13. Houk CP, Kunselman AR, Lee PA. Adequacy of a single unstimulated luteinizing hormone level to diagnose central precocious puberty in girls. Pediatrics 2009;123:e1059ŌĆōe1063. PMID: 19482738.


14. Pasternak Y, Friger M, Loewenthal N, Haim A, Hershkovitz E. The utility of basal serum LH in prediction of central precocious puberty in girls. Eur J Endocrinol 2012;166:295ŌĆō299. PMID: 22084156.


15. Supornsilchai V, Hiranrat P, Wacharasindhu S, Srivuthana S, Aroonparkmongkol S. Basal luteinizing hormone/follicle stimulating hormone ratio in diagnosis of central precocious puberty. J Med Assoc Thai 2003;86(Suppl 2):S145ŌĆōS151. PMID: 12929982.

16. Nam HK, Rhie YJ, Son CS, Park SH, Lee KH. Factors to predict positive results of gonadotropin releasing hormone stimulation test in girls with suspected precocious puberty. J Korean Med Sci 2012;27:194ŌĆō199. PMID: 22323868.



17. Lee HS, Park HK, Ko JH, Kim YJ, Hwang JS. Utility of Basal luteinizing hormone levels for detecting central precocious puberty in girls. Horm Metab Res 2012;44:851ŌĆō854. PMID: 22893259.



18. S├Ėrensen K, Aksglaede L, Petersen JH, Andersson AM, Juul A. Serum IGF1 and insulin levels in girls with normal and precocious puberty. Eur J Endocrinol 2012;166:903ŌĆō910. PMID: 22379117.


19. Biro FM, Lucky AW, Simbartl LA, Barton BA, Daniels SR, Striegel-Moore R, et al. Pubertal maturation in girls and the relationship to anthropometric changes: pathways through puberty. J Pediatr 2003;142:643ŌĆō646. PMID: 12838192.


20. Burt Solorzano CM, McCartney CR. Obesity and the pubertal transition in girls and boys. Reproduction 2010;140:399ŌĆō410. PMID: 20802107.



21. Wang Y. Is obesity associated with early sexual maturation? A comparison of the association in American boys versus girls. Pediatrics 2002;110:903ŌĆō910. PMID: 12415028.


Fig.┬Ā1
Results show considerable overlap between the 2 groups. CPP, central precocious puberty; LH, luteinizing hormone.
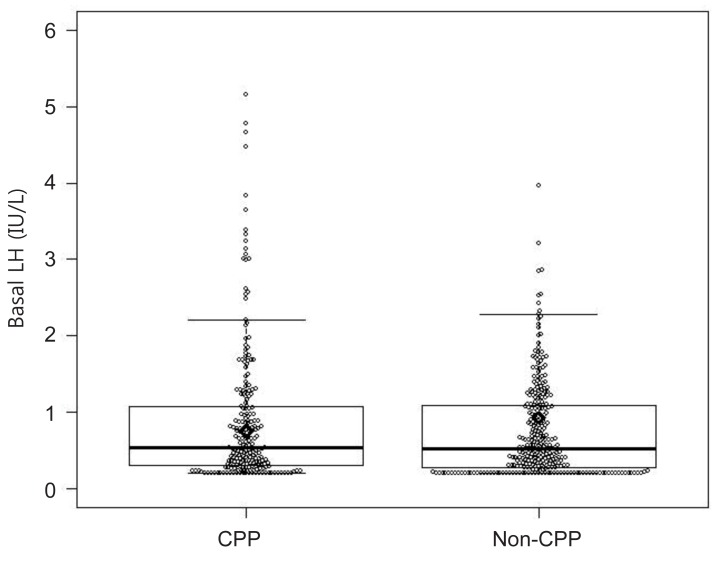
Fig.┬Ā2
The statistically optimal cutoff value of basal luteinizing hormone was 0.22 IU/L, with a sensitivity of 87.8% and a specificity of 20.9% (area under the receiver operating characteristic curve, 0.529).

Fig.┬Ā3
The cutoff value of basal follicle-stimulating hormone was 2.30 IU/L, with a sensitivity of 76.4% and a specificity of 65.9% (area under the receiver operating characteristic curve, 0.780).
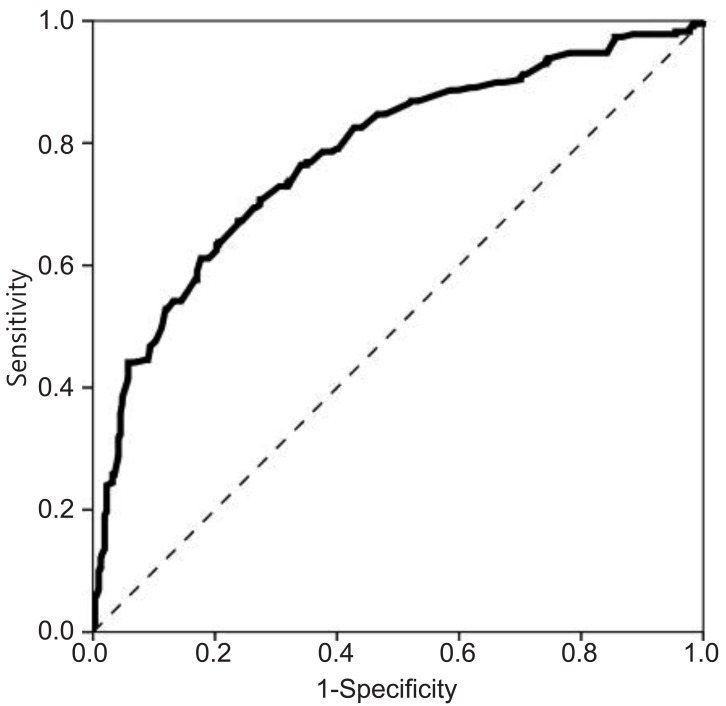
Fig.┬Ā4
The cutoff value of basal estradiol was 8.30 pg/mL, with a sensitivity of 53.3% and a specificity of 65.6% (area under the receiver operating characteristic curve, 0.615).
Table┬Ā1.
Clinical and biochemical parameters in patients with precocious puberty
| Characteristic | CPP (n=229) | Non-CPP (n=311) | P-value |
|---|---|---|---|
| Height (cm) | 132.4┬▒6.4 | 129.7┬▒7.0 | ’╝£0.001 |
| Height SDS | 0.92┬▒0.89 | 0.84┬▒0.95 | 0.340 |
| Yearly growth rate (cm/yr) | 8.88┬▒2.91 | 6.36┬▒2.20 | ’╝£0.001 |
| Bone age (yr) | 10.11┬▒0.64 | 9.74┬▒0.97 | ’╝£0.001 |
| Chronological age (yr) | 8.30┬▒0.69 | 7.96┬▒0.91 | ’╝£0.001 |
| BAŌĆōCA (yr) | 1.81┬▒0.63 | 1.78┬▒0.72 | 0.590 |
| ΔBA/ΔCAa) | 1.16±0.94 | 0.99±0.85 | 0.430 |
| Weight (kg) | 29.87┬▒5.02 | 30.19┬▒5.84 | 0.510 |
| Weight SDS | 0.53┬▒0.77 | 0.79┬▒0.89 | ’╝£0.001 |
| Body mass index (kg/m2) | 16.99┬▒2.05 | 17.89┬▒2.54 | ’╝£0.001 |
| Body mass index SDS | 0.09┬▒0.92 | 0.54┬▒1.04 | ’╝£0.001 |
| Tanner stage | 2.50┬▒0.58 | 2.29┬▒0.56 | ’╝£0.001 |
| Basal LH (IU/L) | 0.93┬▒1.07 | 0.75┬▒0.61 | 0.020 |
| Basal FSH (IU/L) | 3.53┬▒1.84 | 2.06┬▒1.06 | ’╝£0.001 |
| Basal LH/FSH ratio | 0.36┬▒0.63 | 0.47┬▒0.58 | 0.030 |
| Basal estradiol (pg/mL) | 10.59┬▒4.44 | 9.09┬▒2.93 | 0.001 |
| IGF-I (ng/mL) | 270.5┬▒85.6 | 231.5┬▒71.4 | ’╝£0.001 |
| IGF-I SDS | 0.30┬▒0.94 | -0.06┬▒0.80 | ’╝£0.001 |
| IGFBP-3 (┬Ąg/mL) | 2.65┬▒0.53 | 2.60┬▒0.48 | 0.300 |
| IGFBP-3 SDS | -0.50┬▒0.98 | -0.51┬▒0.89 | 0.880 |
| Peak LH (IU/L) | 15.73┬▒13.66 | 3.25┬▒1.01 | ’╝£0.001 |
| Peak FSH (IU/L) | 18.47┬▒7.66 | 15.98┬▒5.99 | ’╝£0.001 |
Table┬Ā2.
Binary logistic regression analysis of the parameters related to GnRH stimulation test results
- TOOLS
-
METRICS

-
- 17 Crossref
- 9,826 View
- 129 Download
- Related articles in APEM





