 |
 |
- Search
| Ann Pediatr Endocrinol Metab > Volume 29(2); 2024 > Article |
|
Abstract
Purpose
Methods
Results
Notes
Funding
This work was supported by the Institute of Research and Development, Walailak University, Thailand under contact no. WU56108.
ACKNOWLEDGMENTS
Fig. 1.
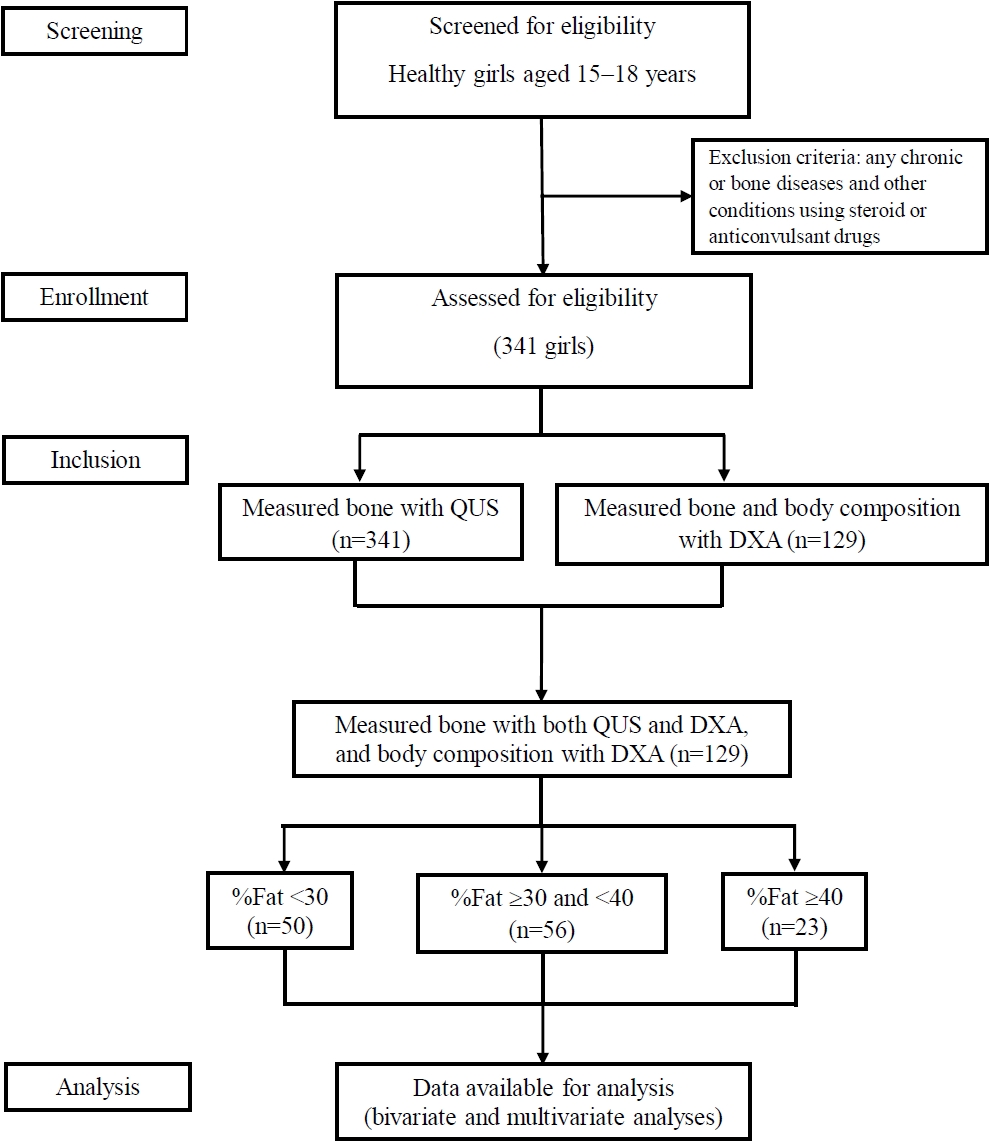
Table 1.
| Variable | Total (n=129) | Group 1 (n=50) | Group 2 (n=56) | Group 3 (n=23) |
P-value† |
P-value‡ |
||
|---|---|---|---|---|---|---|---|---|
| Groups 1, 2, and 3 | Groups 1 and 2 | Groups 1 and 3 | Groups 2 and 3 | |||||
| Anthropometric | ||||||||
| BW (kg) | 53.05±11.56 | 44.29±3.94 | 54.11±8.96 | 69.51±9.06 | <0.001 | <0.001 | <0.001 | <0.001 |
| Height (m) | 1.59±0.06 | 1.58±0.05 | 1.59±0.06 | 1.59±0.07 | 0.703 | 0.911 | 0.680 | 0.857 |
| BMI (kg/m2) | 21.06±4.33 | 17.69±1.38 | 21.51±3.49 | 27.28±2.73 | <0.001 | <0.001 | <0.001 | <0.001 |
| WC (cm) | 73.18±9.54 | 66.52±4.73 | 73.43±7.55 | 87.04±5.95 | <0.001 | <0.001 | <0.001 | <0.001 |
| Body composition | ||||||||
| LBM (g/cm2) | 6.66±0.48 | 6.76±0.46 | 6.61±0.49 | 6.59±0.47 | 0.195 | 0.231 | 0.353 | 0.993 |
| LM (kg) | 31.06±4.84 | 29.06±3.40 | 31.19±5.00 | 35.09±4.67 | <0.001 | 0.037 | <0.001 | 0.001 |
| LMI (kg/m2) | 12.32±1.67 | 11.60±1.17 | 12.38±1.81 | 13.76±1.29 | <0.001 | 0.024 | <0.001 | 0.001 |
| %Lean | 63.93±6.74 | 70.77±2.50 | 62.11±2.65 | 53.52±2.27 | <0.001 | <0.001 | <0.001 | <0.001 |
| BFM (g/cm2) | 3.67±1.23 | 2.52±0.30 | 3.89±0.63 | 5.64±0.60 | <0.001 | <0.001 | <0.001 | <0.001 |
| FM (kg) | 17.35±7.78 | 10.78±1.81 | 17.99±4.70 | 30.07±4.65 | <0.001 | <0.001 | <0.001 | <0.001 |
| FMI (kg/m2) | 6.87±3.01 | 4.30±0.65 | 7.14±1.85 | 11.80±1.55 | <0.001 | <0.001 | <0.001 | <0.001 |
| %Fat | 32.71±7.02 | 25.61±2.59 | 34.61±2.82 | 43.52±2.35 | <0.001 | <0.001 | <0.001 | <0.001 |
| %Bone | 3.35±0.37 | 3.63±0.28 | 3.28±0.25 | 2.91±0.25 | <0.001 | <0.001 | <0.001 | <0.001 |
| QUS parameters | ||||||||
| Radius | ||||||||
| SOS (m/sec) | 3,902.78±104.70 | 3,925.94±93.12 | 3,885.63±99.13 | 3,894.22±133.61 | 0.128 | 0.117 | 0.448 | 0.940 |
| z-score | -0.69±1.88 | -0.34±0.80 | -1.00±2.61 | -0.66±1.25 | 0.201 | 0.173 | 0.780 | 0.745 |
| Tibia | ||||||||
| SOS (m/sec) | 3763.58±119.51 | 3799.86±121.21 | 3755.70±110.61 | 3703.91±113.66 | 0.004 | 0.125 | 0.004 | 0.170 |
| z-score | 0.01±1.11 | 0.31±1.16 | -0.05±1.02 | -0.50±1.05 | 0.013 | 0.208 | 0.011 | 0.224 |
| DXA parameters | ||||||||
| Lumbar spine | ||||||||
| BMD (g/cm2) | 0.97±0.13 | 0.93±0.11 | 0.98±0.12 | 1.03±0.14 | 0.004 | 0.069 | 0.004 | 0.281 |
| BMC (g) | 47.19±8.34 | 45.03±6.43 | 47.36±8.01 | 51.47±11.06 | 0.008 | 0.306 | 0.006 | 0.104 |
| z-score | 0.41±1.10 | 0.01±0.97 | 0.55±1.04 | 0.92±1.25 | 0.002 | 0.028 | 0.003 | 0.339 |
| Whole body | ||||||||
| BMD (g/cm2) | 0.85±0.09 | 0.82±0.09 | 0.86±0.07 | 0.92±0.09 | <0.001 | 0.056 | <0.001 | 0.003 |
| BMC (g) | 1,376.20±261.45 | 1,218.75±159.13 | 1,386.66±205.20 | 1,693.02±272.91 | <0.001 | <0.001 | <0.001 | <0.001 |
| z-score | -0.74±0.64 | -0.98±0.64 | -0.72±0.52 | -0.26±0.61 | <0.001 | 0.060 | <0.001 | 0.005 |
Values are presented as mean±standard deviation.
Group 1, %fat <30; group 2, %fat ≥30 and <40; group 3, %fat ≥40; BW, body weight; BMI, body mass index; WC, waist circumference; LBM, lean body mass; LM, lean mass; LMI, lean mass index; %Lean, body lean percentage; BFM, body fat mass; FM, fat mass; FMI, fat mass index; %Fat, body fat percentage; %Bone, body bone percentage; QUS, quantitative ultrasound; SOS, speed of sound; BMD, bone mineral density; BMC, bone mineral content.
Table 2.
| Variable |
Bone sites |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
Radius |
Tibia |
Lumbar spine |
Whole body |
||||||||
| SOS (m/sec) | z-score | SOS (m/sec) | z-score | BMD (g/cm2) | BMC (g) | z-score | BMD (g/cm2) | BMC (g) | z-score | %Bone | |
| Anthropometric | |||||||||||
| BW (kg) | -0.066 | -0.090 | -0.296** | -0.272** | 0.501*** | 0.562*** | 0.509*** | 0.640*** | 0.882*** | 0.629*** | -0.717*** |
| Height (m) | 0.104 | 0.057 | 0.077 | 0.046 | 0.213* | 0.418*** | 0.227** | 0.180* | 0.436*** | 0.209* | -0.025 |
| BMI (kg/m2) | -0.103 | -0.113 | -0.351*** | -0.313*** | 0.438*** | 0.430*** | 0.442*** | 0.606*** | 0.766*** | 0.585*** | -0.751*** |
| WC (cm) | -0.074 | -0.137 | -0.267** | -0.259** | 0.363*** | 0.415*** | 0.364*** | 0.506*** | 0.696*** | 0.482*** | -0.755*** |
| Body composition | |||||||||||
| LBM (g/cm2) | 0.069 | -0.080 | -0.131 | -0.117 | 0.438*** | 0.499*** | 0.420*** | 0.487*** | 0.403*** | 0.475*** | -0.033 |
| LM (kg) | 0.075 | -0.032 | -0.207* | -0.190* | 0.536*** | 0.653*** | 0.538*** | 0.618*** | 0.815*** | 0.609*** | -0.506*** |
| LMI (kg/m2) | 0.027 | -0.069 | -0.279** | -0.242** | 0.492*** | 0.511*** | 0.487*** | 0.609*** | 0.688*** | 0.582*** | -0.562*** |
| %Lean | 0.128 | 0.113 | 0.349*** | 0.316*** | -0.285** | -0.287** | -0.300** | -0.433*** | -0.659*** | -0.417*** | 0.761*** |
| BFM (g/cm2) | -0.110 | -0.131 | -0.383*** | -0.348*** | 0.364*** | 0.389*** | 0.375*** | 0.532*** | 0.752*** | 0.514*** | -0.785*** |
| FM (kg) | -0.063 | -0.097 | -0.326*** | -0.304*** | 0.404*** | 0.467*** | 0.415*** | 0.561*** | 0.818*** | 0.545*** | -0.753*** |
| FMI (kg/m2) | -0.076 | -0.106 | -0.360*** | -0.331*** | 0.359*** | 0.390*** | 0.367*** | 0.537*** | 0.752*** | 0.516*** | -0.772*** |
| %Fat | -0.131 | -0.116 | -0.350*** | -0.319*** | 0.270** | 0.277** | 0.285** | 0.417*** | 0.651*** | 0.401*** | -0.783*** |
| %Bone | 0.176* | 0.137 | 0.261** | 0.267** | 0.027 | -0.041 | 0.020 | -0.059 | -0.381*** | -0.038 | 1.000 |
SOS, speed of sound; BMD, bone mineral density; BMC, bone mineral content; %Bone, body bone percentage; BW, body weight; BMI, body mass index; WC, waist circumference; LBM, lean body mass; LM, lean mass; LMI, lean mass index; %Lean, body lean percentage; BFM, body fat mass; FM, fat mass; FMI, fat mass index; %Fat, body fat percentage.
Table 3.
| Variable |
Bone sites |
||||||||
|---|---|---|---|---|---|---|---|---|---|
|
Tibia |
Lumbar spine |
Whole body |
|||||||
| SOS (m/sec) | z-score | BMD (g/cm2) | BMC (g) | z-score | BMD (g/cm2) | BMC (g) | z-score | %Bone | |
| Anthropometric | |||||||||
| BW (kg) | -0.391** | -0.339* | 0.372** | 0.507*** | 0.379** | 0.468** | 0.781*** | 0.475*** | -0.228 |
| Height (m) | -0.077 | -0.100 | -0.066 | 0.167 | -0.059 | 0.059 | 0.439*** | 0.074 | -0.129 |
| BMI (kg/m2) | -0.388** | -0.308* | 0.490*** | 0.439** | 0.490*** | 0.496*** | 0.531*** | 0.490*** | -0.155 |
| WC (cm) | -0.076 | -0.118 | -0.054 | -0.043 | -0.046 | 0.062 | 0.087 | 0.056 | -0.365** |
| Body composition | |||||||||
| LBM (g/cm2) | -0.330* | -0.272 | 0.597*** | 0.532*** | 0.603*** | 0.621*** | 0.658*** | 0.619*** | 0.130 |
| LM (kg) | -0.320* | -0.257 | 0.415** | 0.495*** | 0.423** | 0.474** | 0.728*** | 0.459** | -0.163 |
| LMI (kg/m2) | -0.321* | -0.232 | 0.537*** | 0.467** | 0.541*** | 0.524*** | 0.561*** | 0.493*** | -0.100 |
| %Lean | 0.092 | 0.115 | 0.212 | 0.085 | 0.236 | 0.118 | 0.011 | 0.136 | 0.273 |
| BFM (g/cm2) | -0.287* | -0.285* | -0.004 | 0.127 | -0.031 | 0.110 | 0.273 | 0.091 | -0.348* |
| FM (kg) | -0.274 | -0.254 | 0.038 | 0.201 | 0.020 | 0.149 | 0.411** | 0.123 | -0.391** |
| FMI (kg/m2) | -0.289* | -0.259 | 0.080 | 0.164 | 0.057 | 0.153 | 0.279* | 0.115 | -0.389** |
| %Fat | -0.100 | -0.125 | -0.262 | -0.128 | -0.286* | -0.180 | -0.049 | -0.198 | -0.370** |
| %Bone | 0.097 | 0.125 | 0.534*** | 0.435** | 0.536*** | 0.618*** | 0.363** | 0.625*** | 1.000 |
%Fat, body fat percentage; SOS, speed of sound; BMD, bone mineral density; BMC, bone mineral content; %Bone, body bone percentage; BW, body weight; BMI, body mass index; WC, waist circumference; LBM, lean body mass; LM, lean mass; LMI, lean mass index; %Lean, body lean percentage; BFM, body fat mass; FM, fat mass; FMI, fat mass index.
Table 4.
| Variable |
Bone sites |
||||||||
|---|---|---|---|---|---|---|---|---|---|
|
Tibia |
Lumbar spine |
Whole body |
|||||||
| SOS (m/sec) | z-score | BMD (g/cm2) | BMC (g) | z-score | BMD (g/cm2) | BMC (g) | z-score | %Bone | |
| Anthropometric | |||||||||
| BW (kg) | -0.154 | -0.110 | 0.491*** | 0.616*** | 0.486*** | 0.721*** | 0.872*** | 0.715*** | -0.568*** |
| Height (m) | 0.126 | 0.106 | 0.136 | 0.406** | 0.160 | 0.133 | 0.425** | 0.196 | 0.108 |
| BMI (kg/m2) | -0.226 | -0.173 | 0.431** | 0.433** | 0.414** | 0.672*** | 0.681*** | 0.633*** | -0.632** |
| WC (cm) | -0.125 | -0.099 | 0.410** | 0.524*** | 0.388** | 0.629*** | 0.687*** | 0.591*** | -0.596** |
| Body composition | |||||||||
| LBM (g/cm2) | -0.231 | -0.170 | 0.502*** | 0.649*** | 0.490*** | 0.712*** | 0.752*** | 0.693*** | -0.426*** |
| LM (kg) | -0.118 | -0.084 | 0.475*** | 0.663*** | 0.474*** | 0.659*** | 0.855*** | 0.662*** | -0.466*** |
| LMI (kg/m2) | -0.198 | -0.153 | 0.439** | 0.501*** | 0.424*** | 0.648*** | 0.700*** | 0.614*** | -0.575*** |
| %Lean | 0.317* | 0.292* | -0.309* | -0.315* | -0.298* | -0.493*** | -0.529*** | -0.445** | 0.621** |
| BFM (g/cm2) | -0.324* | -0.278* | 0.439** | 0.502*** | 0.426** | 0.671*** | 0.714*** | 0.627*** | -0.691*** |
| FM (kg) | -0.228 | -0.219 | 0.405** | 0.554*** | 0.398** | 0.647*** | 0.786*** | 0.623*** | -0.559*** |
| FMI (kg/m2) | -0.279* | -0.267* | 0.363** | 0.438** | 0.348** | 0.618*** | 0.664*** | 0.572*** | -0.592*** |
| %Fat | -0.306* | -0.285* | 0.279* | 0.294* | 0.267* | 0.470*** | 0.518*** | 0.422** | -0.675*** |
| %Bone | 0.093 | 0.110 | 0.132 | 0.029 | 0.146 | -0.060 | -0.209 | -0.026 | 1.000 |
%Fat, body fat percentage; SOS, speed of sound; BMD, bone mineral density; BMC, bone mineral content; %Bone, body bone percentage; BW, body weight; BMI, body mass index; WC, waist circumference; LBM, lean body mass; LM, lean mass; LMI, lean mass index; %Lean, body lean percentage; BFM, body fat mass; FM, fat mass; FMI, fat mass index.
Table 5.
| Variable |
Bone sites |
||||||
|---|---|---|---|---|---|---|---|
|
Lumbar spine |
Whole body |
||||||
| BMD (g/cm2) | BMC (g) | z-score | BMD (g/cm2) | BMC (g) | z-score | %Bone | |
| Anthropometric | |||||||
| BW (kg) | 0.554** | 0.591** | 0.544** | 0.515* | 0.731*** | 0.489* | -0.290 |
| Height (m) | 0.689*** | 0.686*** | 0.693*** | 0.434* | 0.720*** | 0.431* | 0.196 |
| BMI (kg/m2) | 0.171 | 0.224 | 0.158 | 0.326 | 0.378 | 0.299 | -0.509* |
| WC (cm) | 0.255 | 0.319 | 0.222 | 0.088 | 0.190 | 0.041 | -0.582** |
| Body composition | |||||||
| LBM (g/cm2) | 0.381 | 0.476* | 0.345 | 0.335 | 0.428* | 0.279 | -0.334 |
| LM (kg) | 0.600** | 0.676*** | 0.583** | 0.452* | 0.688*** | 0.423* | -0.233 |
| LMI (kg/m2) | 0.281 | 0.382 | 0.255 | 0.287 | 0.371 | 0.249 | -0.461* |
| %Lean | 0.082 | 0.025 | 0.040 | -0.129 | -0.176 | -0.182 | 0.138 |
| BFM (g/cm2) | 0.137 | 0.256 | 0.145 | 0.366 | 0.436* | 0.373 | -0.298 |
| FM (kg) | 0.481* | 0.577** | 0.494* | 0.521* | 0.741*** | 0.529** | -0.278 |
| FMI (kg/m2) | 0.138 | 0.253 | 0.152 | 0.339 | 0.427* | 0.351 | -0.434* |
| %Fat | -0.140 | -0.064 | -0.106 | 0.039 | 0.110 | 0.088 | -0.263 |
| %Bone | 0.404 | 0.269 | 0.434* | 0.554** | 0.372 | 0.573** | 1.000 |
%Fat, body fat percentage; BMD, bone mineral density; BMC, bone mineral content; %Bone, body bone percentage; BW, body weight; BMI, body mass index; WC, waist circumference; LBM, lean body mass; LM, lean mass; LMI, lean mass index; %Lean, body lean percentage; BFM, body fat mass; FM, fat mass; FMI, fat mass index.
Table 6.
| Bone sites | Dependent variables | Predictive factors | β | P-value | R2 |
|---|---|---|---|---|---|
| Group 1, %Fat <30 | |||||
| Lumbar spine | BMD (g/cm2) | LBM (g/cm2) | 0.597 | <0.001 | 0.357*** |
| BMC (g) | LBM (g/cm2) | 0.532 | <0.001 | 0.283*** | |
| z-score | LBM (g/cm2) | 0.603 | <0.001 | 0.364*** | |
| Whole body | BMD (g/cm2) | LBM (g/cm2) | 0.621 | <0.001 | 0.386*** |
| BMC (g) | BW (kg) | 0.603 | <0.001 | 0.665*** | |
| LBM (g/cm2) | 0.295 | 0.007 | |||
| z-score | LBM (g/cm2) | 0.619 | <0.001 | 0.383*** | |
| %Bone | FM (kg) | -0.391 | 0.005 | 0.153** | |
| Group 2, %Fat ≥30 & <40 | |||||
| Tibia | SOS (m/sec) | BFM (g/cm2) | -0.324 | 0.015 | 0.105* |
| z-score | %Lean | 0.292 | 0.029 | 0.085* | |
| Lumbar spine | BMD (g/cm2) | LBM (g/cm2) | 0.502 | <0.001 | 0.252*** |
| BMC (g) | LM (kg) | 0.663 | <0.001 | 0.439*** | |
| z-score | LBM (g/cm2) | 0.490 | <0.001 | 0.240*** | |
| Whole body | BMD (g/cm2) | BW (kg) | 0.426 | 0.003 | 0.583*** |
| LBM (g/cm2) | 0.387 | 0.007 | - | ||
| BMC (g) | BW (kg) | 0.638 | <0.001 | 0.822*** | |
| Height (m) | 0.218 | 0.001 | - | ||
| LBM (g/cm2) | 0.230 | 0.014 | - | ||
| z-score | BW (kg) | 0.447 | 0.002 | 0.563*** | |
| LBM (g/cm2) | 0.351 | 0.016 | - | ||
| %Bone | BFM (g/cm2) | -0.691 | <0.001 | 0.478*** | |
| Group 3, %Fat ≥40 | |||||
| Lumbar spine | BMD (g/cm2) | Height (m) | 0.689 | <0.001 | 0.475*** |
| BMC (g) | Height (m) | 0.686 | <0.001 | 0.470*** | |
| z-score | Height (m) | 0.693 | <0.001 | 0.481*** | |
| Whole body | BMD (g/cm2) | FM (kg) | 0.521 | 0.011 | 0.272* |
| BMC (g) | FM (kg) | 1.442 | <0.001 | 0.720*** | |
| FMI (kg/m2) | -0.815 | 0.002 | - | ||
| z-score | FM (kg) | 0.529 | 0.009 | 0.280** | |
| %Bone | WC (cm) | -0.582 | 0.004 | 0.338** |







