 |
 |
- Search
| Ann Pediatr Endocrinol Metab > Volume 29(2); 2024 > Article |
|
Abstract
Purpose
Methods
Results
Conclusions
Notes
Funding
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Fig. 1.
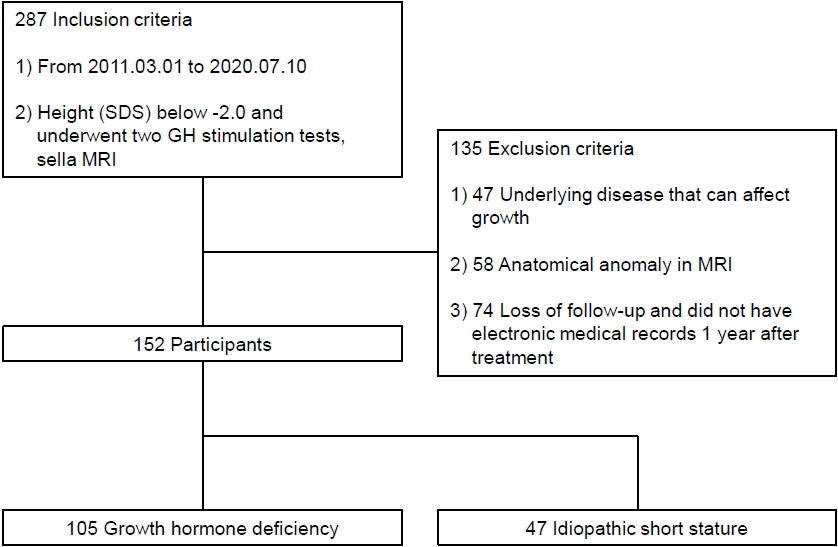
Fig. 2.
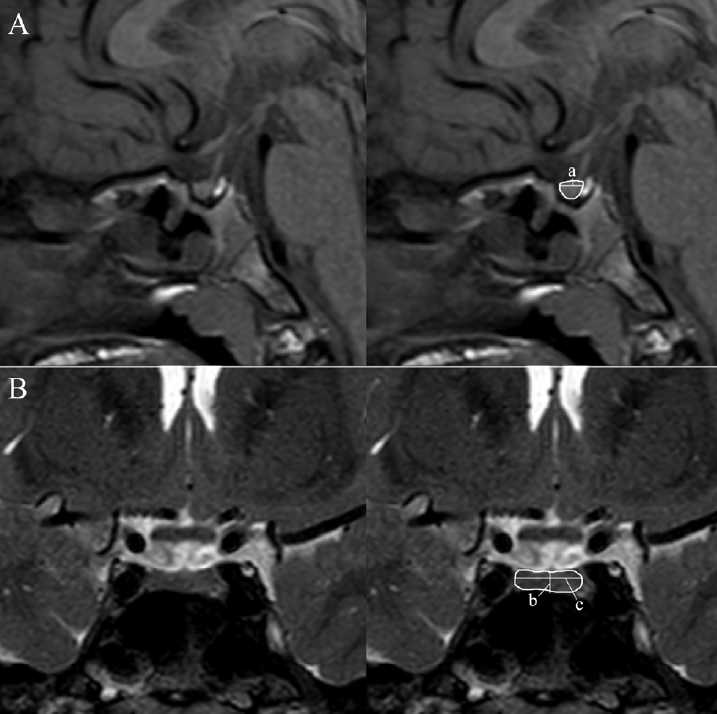
Fig. 3.
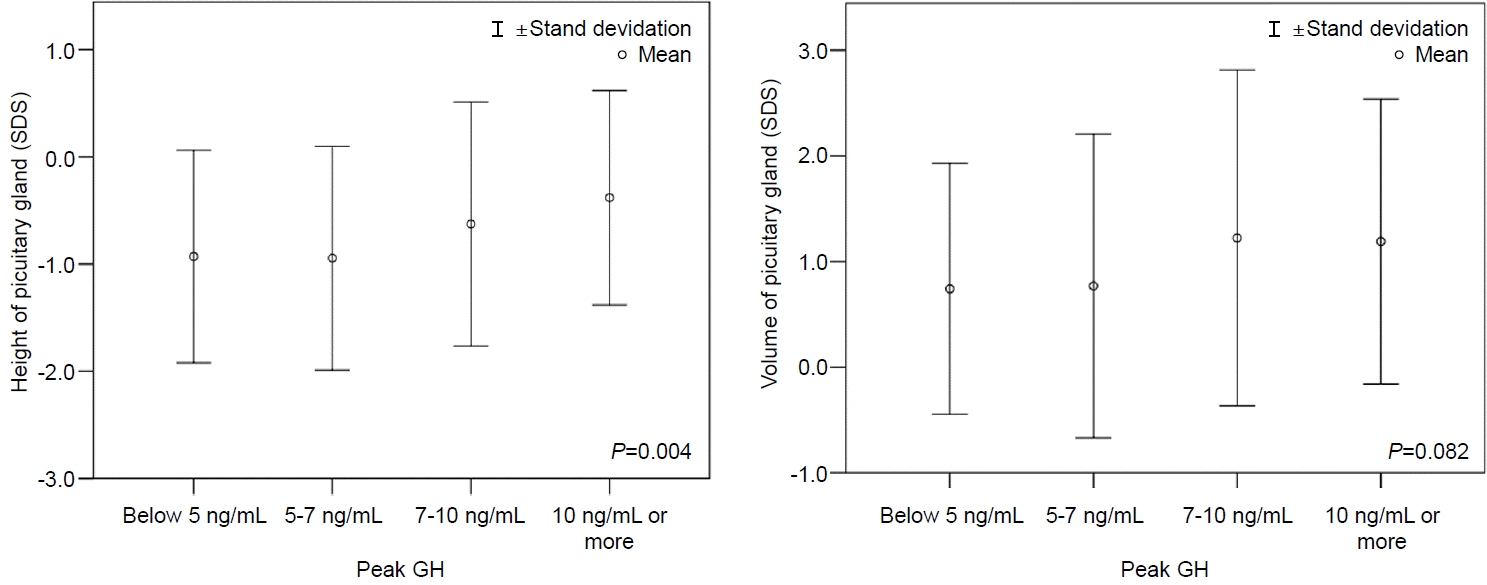
Fig. 4.
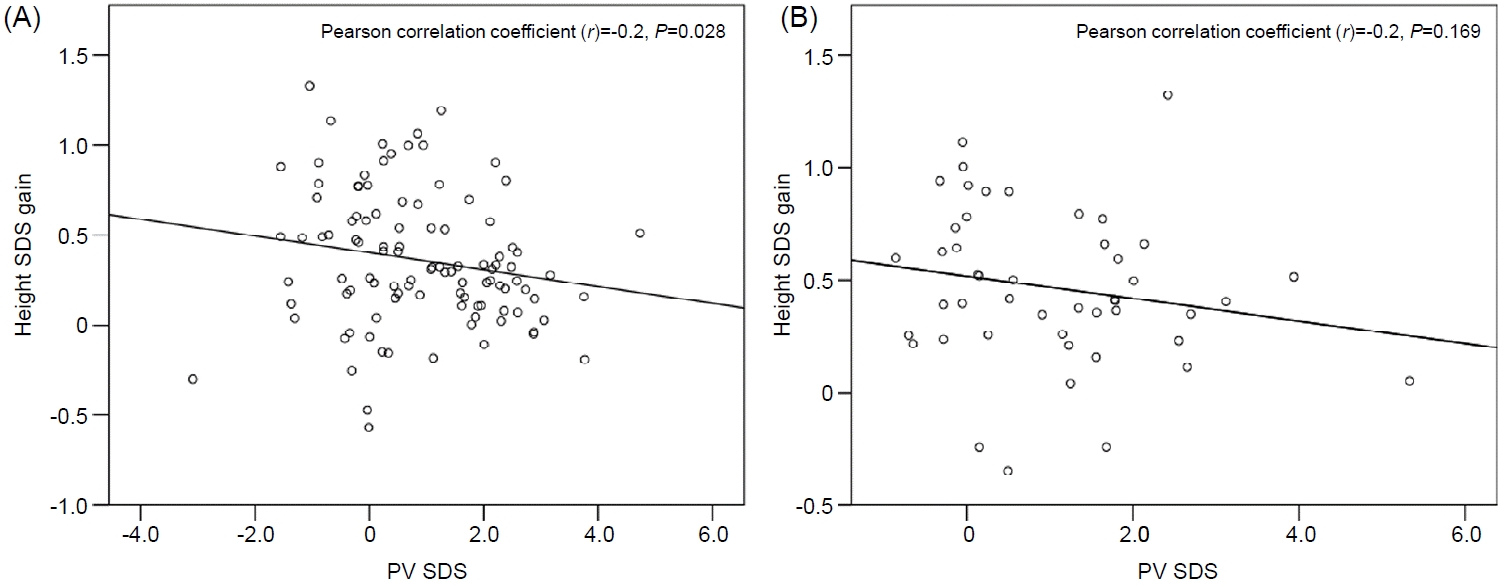
Table 1.
Values are presented as number (%) or mean±standard deviation.
GH, growth hormone; ISS, idiopathic short stature; GHD, growth hormone deficiency; SDS, standard deviation score; MPH, midparental height; IGF-I, insulin-like growth factor I; IGFBP-3, insulin-like growth factor binding protein-3; AP, anteroposterior.
Chi-square test was used for categorical variables, and t-test was used to compare means.
Table 2.
Values are presented as mean±standard deviation.
GH, growth hormone; SDS, standard deviation score; MPH, midparental height; IGF-I, insulin-like growth factor I; IGFBP-3, insulin-like growth factor binding protein-3; AP, anteroposterior.
Good response group was defined as an increase of more than 0.6 in the SDS of the height during the first year of treatment.
t-test was used in single variant analysis, and binary logistic regression was used in multiple linear regression analysis to compare means.
Table 3.
Values are presented as mean±standard deviation.
ISS, idiopathic short stature; SDS, standard deviation score; MPH, midparental height; IGF-I, insulin-like growth factor I; IGFBP-3, insulin-like growth factor binding protein-3; GH, growth hormone; AP, anteroposterior.
Good response group was defined as an increase of more than 0.6 in the SDS of the height during the first year of treatment.
t-test was used in single variant analysis, and binary logistic regression was used in multiple linear regression analysis to compare means.
References
- Related articles in APEM







