|
 |
- Search
| Ann Pediatr Endocrinol Metab > Volume 28(2); 2023 > Article |
|
Abstract
Congenital adrenal hyperplasia (CAH) is a group of autosomally recessive disorders that result from impaired synthesis of glucocorticoid and mineralocorticoid. Most cases (~95%) are caused by mutations in the CYP21A2 gene, which encodes steroid 21-hydroxylase. CAH patients manifest a wide phenotypic spectrum according to their degree of residual enzyme activity. CYP21A2 and its pseudogene (CYP21A1P) are located 30 kb apart in the 6q21.3 region and share approximately 98% of their sequences in the coding region. Both genes are aligned in tandem with the C4, SKT19, and TNX genes, forming 2 segments of the RCCX modules that are arranged as STK19-C4A-CYP21A1P-TNXA-STK19B-C4B-CYP21A2-TNXB. The high sequence homology between the active gene and pseudogene leads to frequent microconversions and large rearrangements through intergenic recombination. The TNXB gene encodes an extracellular matrix glycoprotein, tenascin-X (TNX), and defects in TNXB cause Ehlers-Danlos syndrome. Deletions affecting both CYP21A2 and TNXB result in a contiguous gene deletion syndrome known as CAH-X syndrome. Because of the high homology between CYP21A2 and CYP21A1P, genetic testing for CAH should include an evaluation of copy number variations, as well as Sanger sequencing. Although it poses challenges for genetic testing, a large number of mutations and their associated phenotypes have been identified, which has helped to establish genotype-phenotype correlations. The genotype is helpful for guiding early treatment, predicting the clinical phenotype and prognosis, and providing genetic counseling. In particular, it can help ensure proper management of the potential complications of CAH-X syndrome, such as musculoskeletal and cardiac defects. This review focuses on the molecular pathophysiology and genetic diagnosis of 21-hydroxylase deficiency and highlights genetic testing strategies for CAH-X syndrome.
┬Ę The CYP21A2 gene is located in the major histocompatibility complex class III region on chromosome 6p21.3, approximately 30 kb apart from its pseudogene, CYP21A1P.
┬Ę Molecular diagnosis of 21-hydroxylase deficiency (21-OHD) is challenging because of high sequence homology between CYP21A2 and CYP21A1P, with approximately 98% homology at the exon level and 96% homology at the intron level.
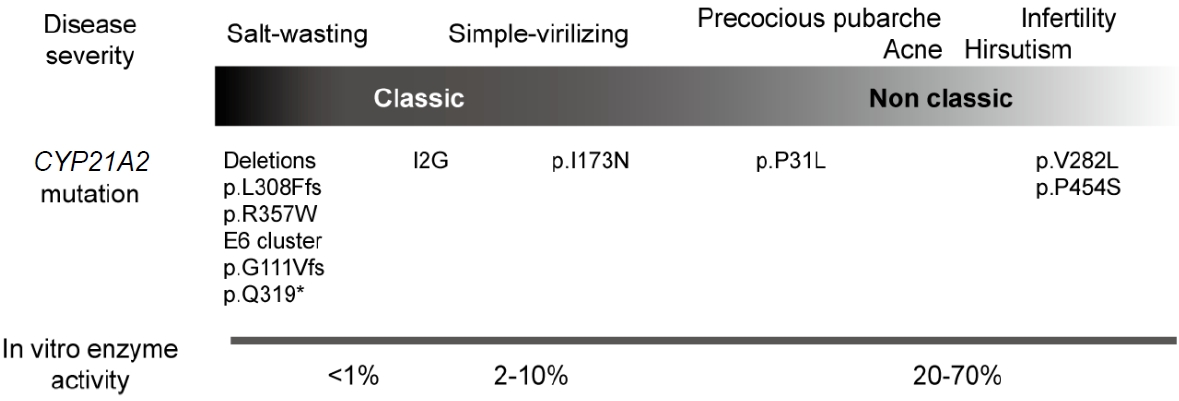
┬Ę The phenotype of 21-OHD varies according to the degree of residual enzyme activity.
┬Ę Genetic diagnosis is helpful in guiding appropriate management and predicting the phenotype and prognosis of patients with 21-OHD.
Congenital adrenal hyperplasia (CAH) is a heterogeneous group of enzyme deficiencies in the steroidogenic pathway of the adrenal cortex that result in impaired synthesis of cortisol and aldosterone by the adrenal glands [1]. Impaired cortisol synthesis causes prolonged elevation of adrenocorticotropic hormone, which leads to overstimulation of the adrenal cortex and subsequent adrenal gland hyperplasia. More than 95% of patients with CAH have steroid 21-hydroxylase deficiency (21-OHD), which is the lack of an enzyme encoded by the CYP21A2 gene [2]. This condition is classified into classic and nonclassic forms based on the residual enzyme activity. The classic form is defined by severely reduced or absent enzyme activity, and it is subdivided into the salt-wasting (SW) form with little or no enzyme activity and the simple virilizing (SV) form with 1% to 5% of residual enzyme activity. Nonclassical CAH (NCCAH) is the mildest form of CAH; it does not cause virilization or a life-threatening adrenal crisis.
The CYP21A2 gene spans 3.35 kb and consists of 10 exons [3,4]. It is located in the major histocompatibility complex class III region of chromosome 6p21.3, and tandem repeats of the active genes and its pseudogenes are aligned within the RCCX module. The tandem repeats often cause complex genomic recombination events between the CYP21A2 and CYP21A1P genes, which are approximately 30 kb apart.
This review describes the molecular mechanism of 21-OHD, its genotype-phenotype correlations, the extended phenotypes of CAH-X syndrome, and genetic testing strategies. Many studies have reported inconsistent nomenclature of mutations in CYP21A2 that result from numbering the initiation codon as 0. In this review, mutations are described according to nomenclature guidelines from the Human Genome Variation Society (https://varnomen.hgvs.org/), in which nucleotide numbering begins with the translation initiation codon (ATG) as the first codon number, and subsequent nucleotides are numbered from the 5' to 3' direction [5].
The CYP21A2 gene is located in a complex organization of genes known as the RCCX module, which is a tandem repeat structure containing a series of functional and nonfunctional genes: serine/threonine kinase 19 (STK19, formerly RP), complement 4 (C4), steroid 21-hydroxylase (CYP21), and tenascin-X (TNX) (Fig. 1A) [3,6]. The RCCX module consists of 2 segments, the short and long modules [7]. The short module contains STK19B, C4B, CYP21A2, and part of TNXB. The long module consists of part of the STK19 gene, the full length of the C4A gene, the CYP21A1P gene, and the TNXA gene. Because of the high sequence homology between the active gene and the pseudogene, microconversions and large rearrangements occur through intergenic recombination [1].
The STK19 gene, which encodes a nuclear serine/threonine kinase protein, was recently proposed to be a regulator of NRAS activity [8]. STK19 phosphorylates NRAS, which facilitates interactions between NRAS and its downstream effectors, increasing the activation of the mitogen-activated protein kinase cascade. C4A (active gene) and C4B (pseudogene), each contain 41 exons and are highly homologous and essential elements of the humoral immune response. The size of the human C4 gene varies from 16 kb to 22 kb due to the integration of the endogenous retroviral sequence (6.7 kb), human endogenous retrovirus-K (HERV-K), in intron 9 of the long C4 gene [9]. Approximately 65% of human C4 gene contains HERV-K viral integration. Although no evidence links HERV-K to human disease, aberrant expression of these retroviral elements is associated with the development of autoimmune diseases [10,11]. Thus, C4 deficiency correlates strongly with autoimmune diseases such as systemic lupus erythematosus [12]. The transcription start site (5'-end) of the CYP21A1P and CYP21A2 genes is located 2,466 bp downstream of both C4A and C4B [13,14].
The CYP21A2 gene encodes 21-hydroxylase, which plays a critical role in the synthesis of the 2 principal steroid hormones, aldosterone and cortisol. CYP21A2 and its pseudogene CYP21A1P share a high degree of sequence homology, with 98% sequence identity in the coding region and 96% in the intronic region [4,15]. The pseudogene is inactivated by several pathogenic variants that prevent the synthesis of a functional protein.
The TNXA and TNXB genes have an opposite transcriptional direction compared with other cluster genes [6,16]. The 3'-ends of the CYP21 genes overlap with the last exons of TNXA and TNXB (Fig. 1A) [6]. The TNXB gene encodes an extracellular matrix glycoprotein, TNX, that consists of 44 exons spanning 68.2 kb. Defects in TNXB cause Ehlers-Danlos syndrome (EDS) [16]. In contrast, TNXA is a truncated pseudogene spanning 4.5 kb and is homologous to TNXB from exons 32 to 44.
A germline copy number variation (CNV) is a fragment of DNA with variable copies that result from duplications or deletions during evolution. Multi-allelic CNVs are genomic segments that exhibit a variable number of copies among individuals. They can affect gene expression, protein function, and phenotypic traits, thereby contributing to genetic and phenotypic heterogeneity. The RCCX module is one of the most complex CNV loci in humans [3]. In Caucasians, RCCX CNV alleles typically have monomodular, bimodular, or trimodular segments, with a prevalence of approximately 17%, 69%, and 14%, respectively (Fig. 1B) [10]. The genetic variations in RCCX CNVs make it difficult to accurately characterize molecular defects in CYP21A2.
As mentioned above, the molecular diagnosis of 21-OHD is challenging because of high sequence homology between CYP21A2 and CYP21A1P. They differ by only 65 nucleotides in their coding and intronic regions [4]. Microconversion of sequence variants from CYP21A1P to CYP21A2 is responsible for nonfunctional protein synthesis in 70%ŌĆō75% of cases (Fig. 2) [2]. Deleterious variants are transferred by small conversions from the pseudogene during meiosis. Microconversion events are caused by major recurrent variants: a splicing mutation (c.293-13A/C>G, also known as I2G), an 8-bp deletion in exon 3 (p.G111Vfs), one nucleotide insertion in exon 7 (p.L308Ffs), 1 nonsense mutation (p.Q319*), 3 missense mutations (p.P31L, p.I173N, and p.R357W), and 1 cluster conversion (p.I237N, p.V238E, and p.M240K) (Fig. 2) [17,18].
In the remaining 25%ŌĆō30% of cases, large gene rearrangements due to unequal crossover during meiosis result in large deletion, duplications, or other contiguous gene deletions [2]. Chimeric CYP21A1P/CYP21A2 genes occur by homologous recombination between these 2 genes. A 26- or 32-kb deletion (depending on the size of C4B) in the region involved in the 3'-end of CYP21A1P, TNXA, and C4B and the 5'-end of CYP21A2 creates a single, nonfunctional chimeric gene (Fig 3A). To date, 9 different chimeric CYP21A1P/CYP21A2 genes have been characterized based on the chimeric junction site (Fig. 3A) [19]. These chimeric genes have been classified into classic and attenuated forms, depending on whether the junction site is upstream or downstream of the I2G mutation [19]. Seven chimeras (CH1, CH2, CH3, CH5, CH6, CH7, and CH8) carrying this splicing mutation (I2G) are associated with a severe SW phenotype [18,19]. In contrast, 2 chimeras (CH4 and CH9) harboring the promoter of CYP21A1P and the p.P31L mutation are associated with an attenuated phenotype [19].
Common major mutations correlate with phenotypes that reflect varying degrees of residual enzyme activity (Fig. 4). Mutations in CYP21A2 are categorized into 4 groups (0, A, B, and C) based on in vitro data according to the level of enzyme activity [23]. Group 0 is caused by null mutations, such as a large deletion, p.L308Ffs, p.Q319*, and p.R357W, that leave no enzyme activity. Group A consists of mutations that cause severely impaired enzyme activity, such as a homozygous or compound heterozygous I2G mutation. Group B reflects either homozygous or compound heterozygous for the p.I173N mutation with group 0 or A variants. Group C consists of milder homozygous or compound heterozygous mutations, such as p.V282L, p.P454S, and p.P31L, which retain 20%ŌĆō60% of residual enzyme activity [1].
Because most patients with 21-OHD harbor compound heterozygous mutations, the allele with the less severe mutation tends to have a greater influence on the clinical phenotype. Severe genotypes (group 0 and A) correlate well with the SW form, ranging from 91%ŌĆō97%; however, this relationship is less strong for the intermediate severity groups (group B and C), which range from 45%ŌĆō57% [24]. For example, patients carrying the p.P31L mutation predominantly have NCCAH, but that mutation has also been reported in the SV type [1,24,25]. This genotype-phenotype discordance could be explained by a genetic modifier. Combined variants of p.P31L and 4 single nucleotide polymorphisms in the promoter region (c.-126C>T, c.-113G>A, c.-110T>C, and c.-103A>G) have been shown to reduce transcriptional activity by 5-fold and enzyme activity by less than 6% [26]. This reduction leads to a more severe phenotype than would be expected on the basis of the genotype alone. Because little research on genetic modifiers in CAH has been done, further studies are needed to fully understand their role. Therefore, genotype-phenotype correlations are not always absolute, and clinical management should be based on clinical features and hormonal data rather than the genotype [1,25,27].
The worldwide incidence of classic CAH, based on neonatal screening, ranges from 1:14,000 to 1:18,000 births [28]. The prevalence of the major mutations varies by ethnicity and geographic region (Table 1). Deletion/conversion, I2G, and p.I173N mutations are the most common in most populations [1]. However, the p.V282L mutation is quite common in Ashkenazi Jews with 21-OHD, and it has rarely been observed in Asians [27]. The I2G and p.V282L mutations are more prevalent in the Middle Eastern population than elsewhere, and the p.Q319* mutation is the most common in the Tunisian population [29,30]. In Korean patients, large deletions are the most common mutation, followed by the I2G and p.I173N [31]. This ethnic diversity and specificity of CYP21A2 mutations could facilitate targeted genetic screening and improve the efficiency of 21-OHD diagnosis.
Deletions affecting both the CYP21A2 and TNXB genes result in a contiguous gene deletion syndrome known as CAH-X syndrome, which is characterized by a hypermobile form of EDS [14,32]. The tenascins are widely-expressed extracellular matrix proteins, and TNX is expressed in the connective tissues, such as skeletal muscle, heart, and blood vessels [16,32]. The role of TNX has been confirmed by Tnx-knockout mice, which have reduced collagen content and connective tissue architecture, suggesting that TNX plays an essential role in regulating collagen deposition in connective tissues [33]. The incidence of CAH-X syndrome is estimated to be 7%ŌĆō15% of patients with 21-OHD [34-36]. However, the exact prevalence of the condition remains unknown because it is often underdiagnosed due to the variability of the phenotype and lack of awareness among physicians.
The TNXA gene is a partially duplicated gene segment that shares a high degree of sequence homology with TNXB from intron 32 to exon 45. TNXA is truncated at the 5' end and harbors a 120-bp deletion causing a frameshift and premature termination of translation [37]. A chimeric TNXA/TNXB gene is caused by unequal gene crossover in the RCCX module, resulting in the complete deletion of the STK19B-C4B-CYP21A2 genes with a fraction of the TNXA and TNXB genes (Fig. 3B) [38]. Three different TNXA/TNXB chimeras have been reported based on the location of the junction site [39]. CAH-X CH1 is caused by a 120 bp deletion in exon 35, which can be detected by a multiplex-ligation dependent probe amplification (MLPA) analysis. Because exons 32ŌĆō34 and exons 36ŌĆō44 are highly homologous, CNV detection in an MLPA analysis is difficult. CAH-X CH2 is characterized by an intact exon 35 and the p.C4058W mutation in exon 40. CAH-X CH3 has a cluster of 3 pseudogene-derived variants: p.R4073H in exon 41, p.D4172N in exon 43, and p.S4175N in exon 43 [38].
To date, the clinical features of more than 50 patients with CAH-X syndrome have been described [34-36,38,40]. Overall, skin laxity and musculoskeletal features, such as joint hypermobility and subluxation, are common. Chronic arthralgia is more prominent in adult patients with CAH-X syndrome than in children. Cardiac abnormalities such as septal defects, cardiac chamber enlargement, or great vessel enlargement occur in about 25% of patients with this condition. These patients also have gastrointestinal abnormalities, including gastroesophageal reflux, hernia, and rectal prolapse. Other findings include early-onset osteoarthritis, scoliosis, pectus excavatum, osteoporosis, bifid or elongated uvula, easy bruising, and delayed wound healing. Patients with biallelic deletion of TNXB have more severe clinical manifestations than those with monoallelic deletion [35].
Molecular genetic testing for 21-OHD is a complex process due to the genomic structure of CYP21A2 and the surrounding region. Various disease-causing mutations include point mutations, large gene rearrangements, multiple mutations in cis, and CNVs of variable sizes. Large gene rearrangements can be detected by Southern blot analyses, real-time quantitative polymerase chain reaction (PCR), and MLPA analyses. Currently, Sanger sequencing combined with an MLPA analysis is commonly used and can detect most mutations.
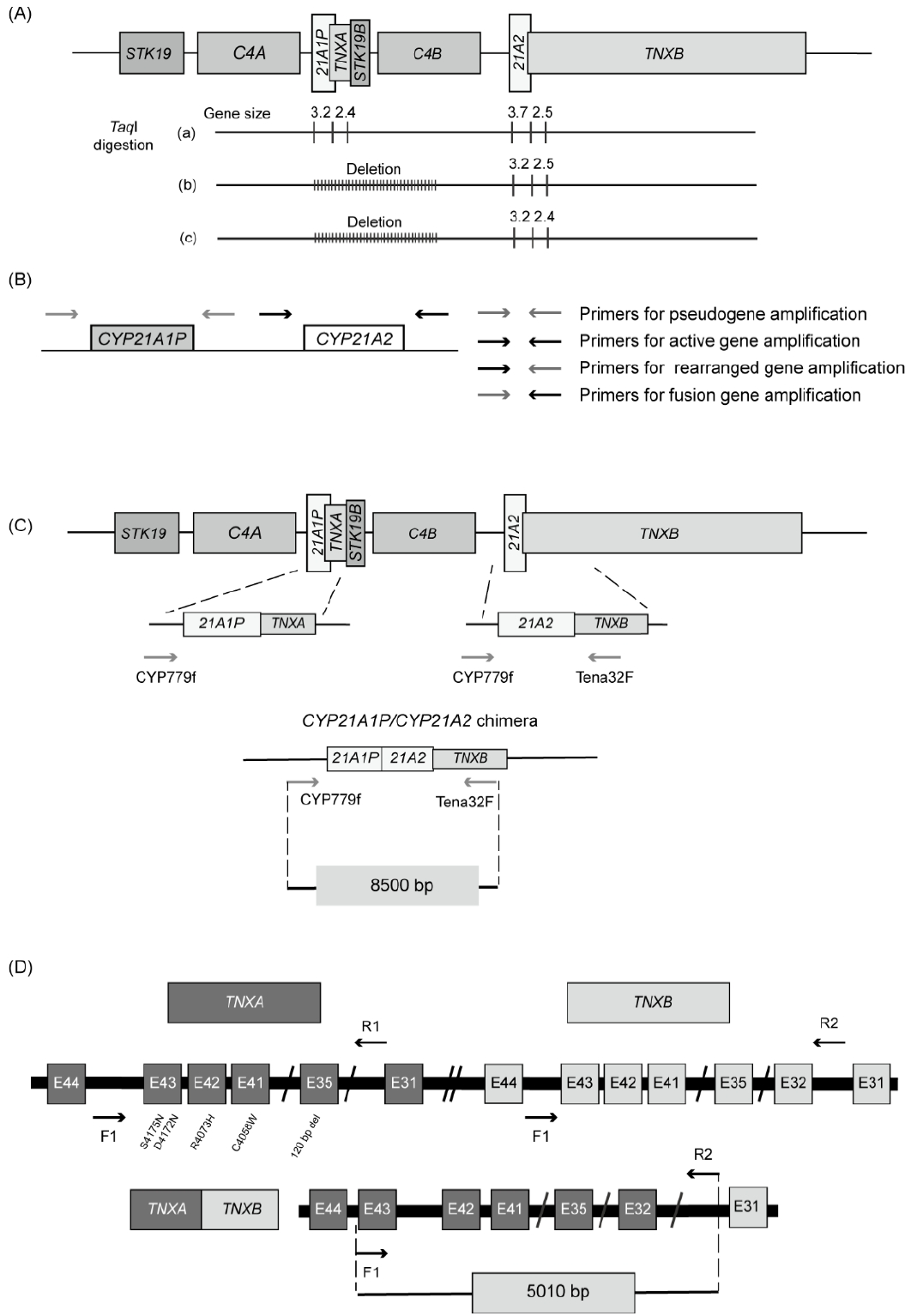
Southern blot analyses were originally used to detect large gene deletions or duplications. This method uses restriction endonucleases that recognize short DNA sequences and cleave double-stranded DNA. The most commonly used restriction endonuclease is TaqI, which recognizes the palindromic DNA sequence of 5'-T^CGA-3', where the caret symbol (^) indicates the cleavage site. This enzyme produces 3.7- and 2.5-kb fragments of intact CYP21A2 and the partial TNXB gene, respectively, and 3.2- and 2.4-kb fragments of CYP21A1P and the partial TNXA gene, respectively (Fig. 5A) [41]. The 3.2- and 3.7-kb fragments are indicative of the CYP21A1P and CYP21A2 genes by their size. Southern blot analyses show that the 3.2-kb fragment without the 3.7-kb fragment represents deletion of the CYP21A2 gene (Fig. 5A). However, this method is time-consuming and requires large amounts of DNA.
Real-time, quantitative PCR is a rapid and sensitive method for detecting gene deletions and duplications. This method uses CYP21A2-specific and internal control gene primer sets and measures the threshold cycle (Ct) values of both the target and control genes. To assess the CYP21A2 copy number, the difference in Ct for CYP21A2 and the internal control gene is calculated and plotted against the logarithm of the CYP21A2/internal control ratio (%). However, some discrepancies with Southern blot analyses can occur due to limited discrimination between 2 and 3 RCCX copies per genome [42].
Sanger sequencing allows the detection of point mutations and small insertions/deletions. PCR with oligonucleotide-specific primers designed for differential amplification of CYP21A2 (3.48 kb) and CYP21A1P (3.49 kb) (Fig. 5B) has been widely used. 31,43) The alleles containing the fusion gene can be detected using a primer set containing CYP21A1P-specific forward and CYP21A2-specific reverse primers. A CYP21A2-specific forward primer and a CYP21A1P-specific reverse primer can be used to detect alleles containing a rearranged gene. PCR products using CYP21A2-specific primer sets are analyzed by automatic direct sequencing to detect point mutations.
When designing primers for PCR-based sequencing, it is crucial to ensure that they are specific for the target gene or region of interest to prevent amplification of the pseudogene. Nonamplification by PCR can result from allelic dropout, which must be considered, as for any other gene [44]. Several single nucleotide polymorphisms in the promoters of both genes can interfere with primer binding, resulting in allelic dropout.
Another well-established technique is long-range PCR for an 8.5-kb fragment using CYP779f/Tena32F primers (Fig. 5C) that encompass the entire CYP21A2 gene and part of TNXB [45]. CYP779f (5'-AGGTGGGCTGTTTTCCTTTCA-3') is a common forward primer for the 5'-untranslated region (UTR) of the CYP21A2 and CYP21A1P genes. The CYP779f primer anneals from c.-799 to c.-779 based on the nucleotide sequences first described by Higashi et al. in 1986 (c.-802 to c.-782 based on NM_000500.9) [3,46]. The reverse primer (Tena32F) (5'-CTGTGCCTGGCTATAGCAAGC-3') anneals specifically to exon 32 of TNXB. This method can be used to detect TNXA/TNXB chimeras in patients with CAH-X syndrome. The amplified PCR products are then subjected to Sanger sequencing.
The MLPA analysis is a robust method that can detect gene deletions, rearrangements, and fusion genes, and it requires only small amounts of DNA. A commercially available CYP21A2-MLPA kit (SALSA MLPA Probemix P050 CAH, MRC Holland, Amsterdam, The Netherlands) is widely used [44]. It contains 4 probes for the CYP21A1P gene and 8 probes for the CYP21A2 gene, enabling it to detect gene deletions, large gene conversions, a single nucleotide variant in the 5'-UTR (c.-113G>A), an 8 bp deletion in exon 3, p.I173N in exon 4, E6 cluster mutations in exon 6, and p.L308Ffs in exon 7. In addition, the probe mix contains 6 probes for the TNXB gene and can detect a 120 bp deletion at the boundary of exon 35 and intron 35 (CAH-X CH1). This method is limited to detecting variants that lie outside the target probe. Despite the challenges associated with genetic testing for 21-OHD, accurate genetic testing (up to 98%) can be achieved using PCR-based sequencing and MLPA [44].
PCR-based detection of chimeric genes and MLPA analyses have been carried out to confirm the diagnosis of CAH-X syndrome [35]. PCR-based detection of chimeric genes has been performed using a mixed-primer strategy: a common forward primer for intron 43 of TNXA and TNXB and a reverse primer for intron 31 of TNXB (Fig. 5D). This produces a 5,010 bp fragment of TNXB, from part of intron 31 to part of intron 43 (Fig. 5D). This PCR product is used as a template for the subsequent sequencing analysis to assess TNXA/TNXB chimeras. CAH-X CH1 alleles are assessed by Sanger sequencing of exon 35 in TNXB, whereas CH2 and CH3 alleles are identified by direct sequencing of exons 40, 41, and 43. An MLPA analysis can detect a 120-bp deletion in TNXB exon 35 that is derived from the TNXA pseudogene. However, that is a laborious process and is not routinely performed. Therefore, the diagnosis of CAH-X syndrome relies primarily on clinical assessment using the Beighton 9-point scale [47].
Recently, next-generation sequencing techniques have been increasingly used in clinical diagnostics, replacing traditional sequencing technologies. Because pseudogenes have high sequence homology, short read sequencing is limited in its ability to accurately map the reads to the correct genomic location. In addition, the complex genomic structure of RCCX module produces structural variations that are difficult to characterize using short-read sequencing technologies [48].
Therefore, long-read sequencing (LRS) technologies have been developed, such as single molecule real time (SMRT) sequencing (Pacific Biosciences, Menlo Park, CA, USA) and Oxford Nanopore sequencing (Oxford Nanopore Technologies, Oxford, UK) [49]. These techniques generate reads long enough to investigate repetitive or homologous regions. Long-range PCR encompassing the full-length of the CYP21A1P/TNXA and CYP21A2/partial TNXB genes that is followed by SMRT sequencing can detect various mutations, including point mutations and small insertions/deletions [50]. Locus-specific PCR using CYP779f/Tena32F followed by SMRT sequencing using the Sequel II platform (Pacific Biosciences) successfully identified pathogenic variants [51]. However, those studies showed that current LRS by PCR of a partial region is still limited in its ability to find CNVs and complex rearrangements. In addition, specialized bioinformatic algorithms are required to accurately detect mutations in highly homologous regions. Advances in bioinformatic tools and reductions in the cost of LRS-based testing will mitigate current challenges in the future.
The CYP21A2 gene is located on the RCCX module, which is a complex, multiallelic, and tandem CNV. This highly homologous gene structure can lead to genetic rearrangement during meiosis. Genetic testing is challenging due to the presence of pseudogenes and complex genomic structures. Recent extensive studies of the RCCX modules have led to a better understanding of the spectrum of TNX-related disorders. Identification of CAH-X syndrome is important to enable early intervention for musculoskeletal and cardiac abnormalities. The combination of clinical and biochemical markers with appropriate genetic testing promotes better outcomes in patients with 21-OHD.
Notes
Fig.┬Ā1.
Organization of the human RCCX module and copy number variations on chromosome 6p21. (A) Schematic representation of the RCCX module featuring 2 segments with the following gene arrangement: STK19 (formerly RP1)-C4A-CYP21A1P-TNXA-STK19B-C4B-CYP21A2-TNXB. The arrow indicates transcriptional orientation. (B) Three different RCCX module arrangements: monomodular, bimodular, and trimodular structures.
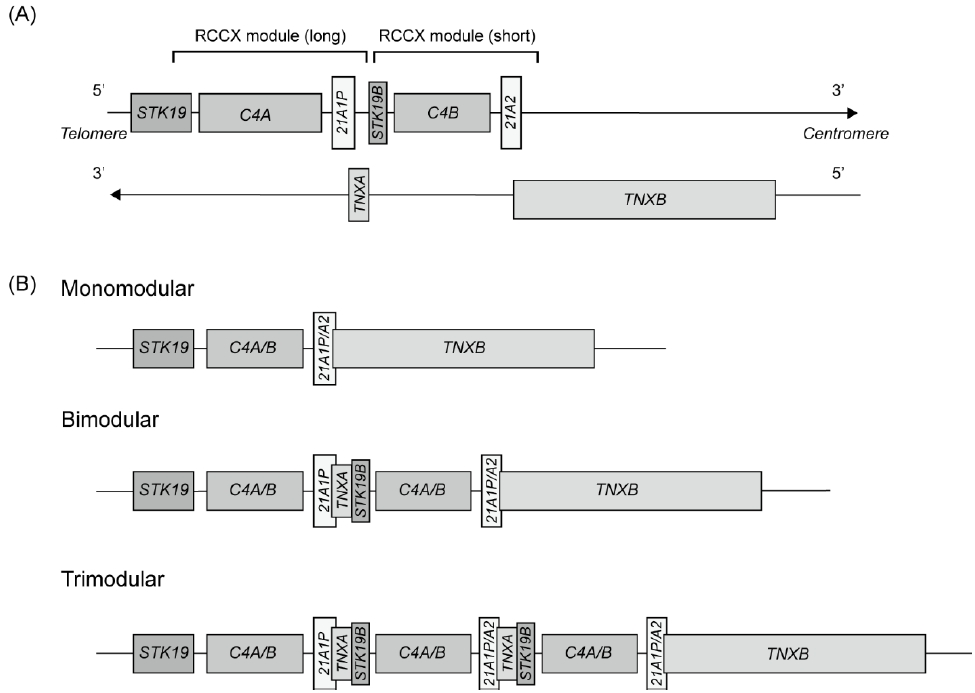
Fig.┬Ā2.
Schematic representation of microconversion events and the location of common mutations within the CYP21A2 gene transferred by gene conversion. NC, nonclassical; SV, simple virilizing; SW, salt-wasting.
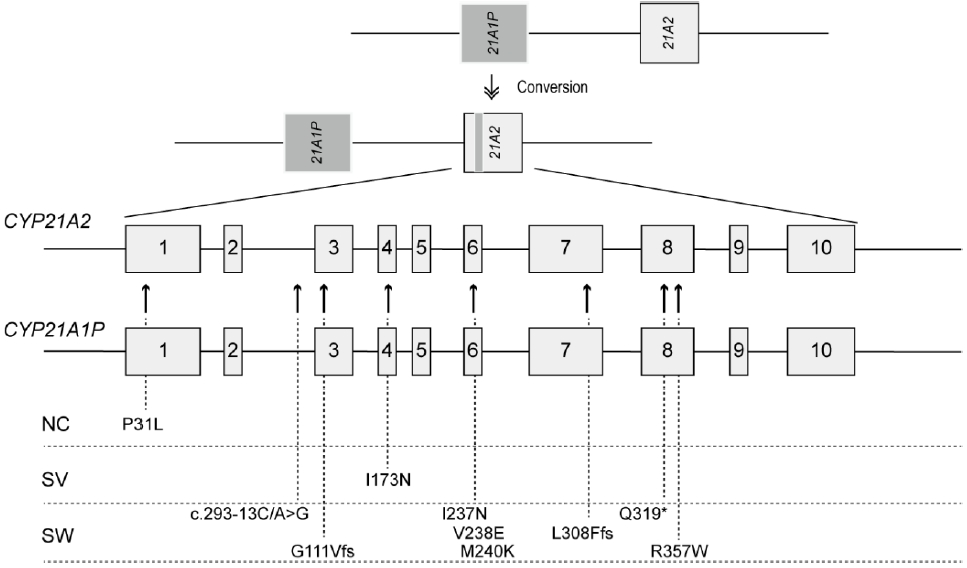
Fig.┬Ā3.
Gene rearrangement due to unequal crossover during meiosis. (A) CYP21A2 gene rearrangement caused by a 30-kb deletion, leading to a CYP21A1P/CYP21A2 chimera. The figure illustrates the 9 types of chimera according to the different junction sites. Seven chimeras harboring the I2G mutation are associated with the classic salt-wasting phenotype, and 2 chimeras carrying a p.P31L mutation exhibit an attenuated phenotype. (B) Formation of a chimeric TNXA/TNXB gene that deletes the CYP21A2 gene. Three different chimeric genes are demonstrated, depending on the junction site.

Fig.┬Ā5.
Schematic representation of a molecular genetic analysis of CYP21A2. (A) Southern blot analysis digested with TaqI restriction endonuclease. This method distinguishes the CYP21A2 (3.7 kb) and CYP21A1P (3.2 kb) genes by fragment size. (B) Sequence-specific PCR amplification of the CYP21A1P and CYP21A2 genes. Two set of primers create 4 possible pairwise reactions to obtain amplicons corresponding to CYP21A1P, CYP21A2, the fusion gene, and rearrangement products.31) (C) Sequencing for a CYP21A1P/CYP21A2 chimera using paired primers, CYP779f/Tena32F. These primers amplify the 8.5-kb fragment. The Tena32F primer is located in a nonduplicated area of TNXB in exon 32, and the CYP779f primer is complementary to the 5ŌĆÖ-end of the CYP21A1P and CYP21A2 genes. (D) Sequencing for a TNXA/TNXB chimera using mixed paired primers. The forward primer (F1) contains complementary sequences for intron 43 of TNXA and TNXB, and the reverse primer (R2) is specific for intron 31 of TNXB.
Table┬Ā1.
Allele frequencies in various regions
References
1. Claahsen-van der Grinten HL, Speiser PW, Ahmed SF, Arlt W, Auchus RJ, Falhammar H, et al. Congenital adrenal hyperplasia-Current insights in pathophysiology, diagnostics, and management. Endocr Rev 2022;43:91ŌĆō159.



2. Pignatelli D, Carvalho BL, Palmeiro A, Barros A, Guerreiro SG, Macut D. The complexities in genotyping of congenital adrenal hyperplasia: 21-hydroxylase deficiency. Front Endocrinol (Lausanne) 2019;10:432.



3. Higashi Y, Yoshioka H, Yamane M, Gotoh O, Fujii-Kuriyama Y. Complete nucleotide sequence of two steroid 21-hydroxylase genes tandemly arranged in human chromosome: a pseudogene and a genuine gene. Proc Natl Acad Sci U S A 1986;83:2841ŌĆō5.



4. White PC, New MI, Dupont B. Structure of human steroid 21-hydroxylase genes. Proc Natl Acad Sci U S A 1986;83:5111ŌĆō5.



5. den Dunnen JT, Dalgleish R, Maglott DR, Hart RK, Greenblatt MS, McGowan-Jordan J, et al. HGVS recommendations for the description of sequence variants: 2016 update. Hum Mutat 2016;37:564ŌĆō9.


6. Morel Y, Bristow J, Gitelman SE, Miller WL. Transcript encoded on the opposite strand of the human steroid 21-hydroxylase/complement component C4 gene locus. Proc Natl Acad Sci U S A 1989;86:6582ŌĆō6.



7. Lee H-H. Chimeric CYP21P/CYP21 and TNXA/TNXB genes in the RCCX module. Mol Genet Metab 2005;84:4ŌĆō8.


8. Yin C, Zhu B, Zhang T, Liu T, Chen S, Liu Y, et al. Pharmacological targeting of STK19 inhibits oncogenic NRAS-driven melanomagenesis. Cell 2019;176:1113ŌĆō27.e16.


9. Tassabehji M, Strachan T, Anderson M, Campbell RD, Collier S, Lako M. Identification of a novel family of human endogenous retroviruses and characterization of one family member, HERV-K(C4), located in the complement C4 gene cluster. Nucleic Acids Res 1994;22:5211ŌĆō7.



10. Blanchong CA, Zhou B, Rupert KL, Chung EK, Jones KN, Sotos JF, et al. Deficiencies of human complement component C4a and C4b and heterozygosity in length variants of RP-C4-CYP21-TNX (Rccx) modules in Caucasians: the load of Rccx genetic diversity on major histocompatibility complexŌĆōassociated disease. J Exp Med 2000;191:2183ŌĆō96.


11. Grandi N, Cadeddu M, Pisano MP, Esposito F, Blomberg J, Tramontano E. Identification of a novel HERV-K(HML10): comprehensive characterization and comparative analysis in non-human primates provide insights about HML10 proviruses structure and diffusion. Mob DNA 2017;8:15.




13. Wijesuriya SD, Zhang G, Dardis A, Miller WL. Transcriptional regulatory elements of the human gene for cytochrome P450c21 (steroid 21-hydroxylase) lie within intron 35 of the linked C4B gene. J Biol Chem 1999;274:38097ŌĆō106.


14. Miller WL, Merke DP. Tenascin-X, Congenital adrenal hHyperplasia, and the CAH-X syndrome. Horm Res Paediatr 2018;89:352ŌĆō61.



15. White PC, Grossberger D, Onufer BJ, Chaplin DD, New MI, Dupont B, et al. Two genes encoding steroid 21-hydroxylase are located near the genes encoding the fourth component of complement in man. Proc Natl Acad Sci U S A 1985;82:1089ŌĆō93.



16. Bristow J, Tee MK, Gitelman SE, Mellon SH, Miller WL. Tenascin-X: a novel extracellular matrix protein encoded by the human XB gene overlapping P450c21B. J Cell Biol 1993;122:265ŌĆō78.




17. Choi JH, Kim GH, Yo o HW. R ecent advances in biochemical and molecular analysis of congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Ann Pediatr Endocrinol Metab 2016;21:1ŌĆō6.



18. Concolino P, Costella A. Congenital adrenal hyperplasia (CAH) due to 21-hydroxylase deficiency: a comprehensive focus on 233 pathogenic variants of CYP21A2 Gene. Mol Diagn Ther 2018;22:261ŌĆō80.



19. Chen W, Xu Z, Sullivan A, Finkielstain GP, Van Ryzin C, Merke DP, et al. Junction site analysis of chimeric CYP21A1P/CYP21A2 genes in 21-hydroxylase deficiency. Clin Chem 2012;58:421ŌĆō30.



20. Billerbeck AE, Mendonca BB, Pinto EM, Madureira G, Arnhold IJ, Bachega TA. Three novel mutations in CYP21 gene in Brazilian patients with the classical form of 21-hydroxylase deficiency due to a founder effect. J Clin Endocrinol Metab 2002;87:4314ŌĆō7.


21. de Carvalho DF, Miranda MC, Gomes LG, Madureira G, Marcondes JAM, Billerbeck AEC, et al. Molecular CYP21A2 diagnosis in 480 Brazilian patients with congenital adrenal hyperplasia before newborn screening introduction. Eur J Endocrinol 2016;175:107ŌĆō16.


22. Loidi L, Quinteiro C, Parajes S, Barreiro J, Lest├│n DG, Cabezas-Agr├Łcola JM, et al. High variability in CYP21A2 mutated alleles in Spanish 21-hydroxylase deficiency patients, six novel mutations and a founder effect. Clin Endocrinol (Oxf) 2006;64:330ŌĆō6.


23. Speiser PW, Dupont J, Zhu D, Serrat J, Buegeleisen M, Tusie-Luna MT, et al. Disease expression and molecular genotype in congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J Clin Invest 1992;90:584ŌĆō95.



24. Riedl S, R├Čhl FW, Bonfig W, Br├żmswig J, Richter-Unruh A, Fricke-Otto S, et al. Genotype/phenotype correlations in 538 congenital adrenal hyperplasia patients from Germany and Austria: discordances in milder genotypes and in screened versus prescreening patients. Endocr Connect 2019;8:86ŌĆō94.



25. Kocova M, Anastasovska V, Falhammar H. Clinical outcomes and characteristics of P30L mutations in congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Endocrine 2020;69:262ŌĆō77.




26. Araujo RS, Billerbeck AE, Madureira G, Mendonca BB, Bachega TA. Substitutions in the CYP21A2 promoter explain the simple-virilizing form of 21-hydroxylase deficiency in patients harbouring a P30L mutation. Clin Endocrinol (Oxf) 2005;62:132ŌĆō6.


27. New MI, Abraham M, Gonzalez B, Dumic M, Razzaghy-Azar M, Chitayat D, et al. GenotypeŌĆōphenotype correlation in 1,507 families with congenital adrenal hyperplasia owing to 21-hydroxylase deficiency. Proc Natl Acad Sci U S A 2013;110:2611ŌĆō6.



28. Speiser PW, Arlt W, Auchus RJ, Baskin LS, Conway GS, Merke DP, et al. Congenital adrenal hyperplasia due to steroid 21-hydroxylase deficiency: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 2018;103:4043ŌĆō88.



29. Romdhane L, Kefi R, Azaiez H, Halim NB, Dellagi K, Abdelhak S. Founder mutations in Tunisia: implications for diagnosis in North Africa and Middle East. Orphanet J Rare Dis 2012;7:52.




30. Kharrat M, Tardy Vr, MŌĆÖRad R, Maazoul F, Jemaa LB, Refai╠ł M, et al. Molecular genetic analysis of Tunisian patients with a classic form of 21-hydroxylase deficiency: identification of four novel mutations and high prevalence of Q318X mutation. J Clin Endocrinol Metab 2004;89:368ŌĆō74.


31. Choi JH, Jin HY, Lee BH, Ko JM, Lee JJ, Kim GH, et al. Clinical phenotype and mutation spectrum of the CYP21A2 gene in patients with steroid 21-hydroxylase deficiency. Exp Clin Endocrinol Diabetes 2012;120:23ŌĆō7.


32. Burch GH, Gong Y, Liu W, Dettman RW, Curry CJ, Smith L, et al. TenascinŌĆōX deficiency is associated with EhlersŌĆōDanlos syndrome. Nat Genet 1997;17:104ŌĆō8.



33. Mao JR, Taylor G, Dean WB, Wagner DR, Afzal V, Lotz JC, et al. Tenascin-X deficiency mimics Ehlers-Danlos syndrome in mice through alteration of collagen deposition. Nat Genet 2002;30:421ŌĆō5.



34. Morissette R, Chen W, Perritt AF, Dreiling JL, Arai AE, Sachdev V, et al. Broadening the spectrum of Ehlers Danlos syndrome in patients with congenital adrenal hyperplasia. J Clin Endocrinol Metab 2015;100:E1143ŌĆō52.



35. Merke DP, Chen W, Morissette R, Xu Z, Ryzin CV, Sachdev V, et al. Tenascin-X haploinsufficiency associated with Ehlers-Danlos syndrome in patients with congenital adrenal hyperplasia. J Clin Endocrinol Metab 2013;98:E379ŌĆō87.



36. Lao Q, Brookner B, Merke DP. High-throughput screening for CYP21A1P-TNXA/TNXB chimeric genes responsible for Ehlers-Danlos syndrome in patients with congenital adrenal hyperplasia. J Mol Diagn 2019;21:924ŌĆō31.



37. Gitelman SE, Bristow J, Miller WL. Mechanism and consequences of the duplication of the human C4/P450c21/gene X locus. Mol Cell Biol 1992;12:2124ŌĆō34.



38. Marino R, Garrido NP, Ramirez P, Notarist├®fano G, Moresco A, Touzon MS, et al. Ehlers-Danlos syndrome: Molecular and clinical characterization of TNXA/TNXB chimeras in congenital adrenal hyperplasia. J Clin Endocrinol Metab 2021;106:e2789. ŌĆō802.



39. Carrozza C, Foca L, Paolis ED, Concolino P. Genes and pseudogenes: complexity of the RCCX locus and disease. Front Endocrinol (Lausanne) 2021;12:709758.



40. Lao Q, Mallappa A, Rueda Faucz F, Joyal E, Veeraraghavan P, Chen W, et al. A TNXB splice donor site variant as a cause of hypermobility type EhlersŌĆōDanlos syndrome in patients with congenital adrenal hyperplasia. Mol Genet Genomic Med 2021;9:e1556.



41. Lee HH, Lee YJ, Chao MC. Comparing the Southern blot method and polymerase chain reaction product analysis for chimeric RCCX detection in CYP21A2 deficiency. Anal Biochem 2010;399:293ŌĆō8.


42. Olney RC, Mougey EB, Wang J, Shulman DI, Sylvester JE. Using real-time, quantitative PCR for rapid genotyping of the steroid 21-hydroxylase gene in a North Florida population. J Clin Endocrinol Metab 2002;87:735ŌĆō41.


43. Ravichandran L, Varghese D, R P, S AH, Korula S, Thomas N, et al. Allele-specific and multiplex PCR based tools for cost-effective and comprehensive genetic testing in Congenital Adrenal Hyperplasia. MethodsX 2022;9:101748.



44. Baumgartner-Parzer S, Witsch-Baumgartner M, Hoeppner W. EMQN best practice guidelines for molecular genetic testing and reporting of 21-hydroxylase deficiency. Eur J Hum Genet 2020;28:1341ŌĆō67.




45. Krone N, Arlt W. Genetics of congenital adrenal hyperplasia. Best Pract Res Clin Endocrinol Metab 2009;23:181ŌĆō92.



46. Lee HH, Chang SF, Tsai FJ, Tsai LP, Lin CY. Mutation of IVS2-12A/C>G in combination with 707-714delGAGACTAC in the CYP21 gene is caused by deletion of the C4-CYP21 repeat module with steroid 21-hydroxylase deficiency. J Clin Endocrinol Metab 2003;88:2726ŌĆō9.

47. Beighton P, De Paepe A, Steinmann B, Tsipouras P, Wenstrup RJ. Ehlers-Danlos syndromes: revised nosology, Villefranche, 1997. Ehlers-Danlos National Foundation (USA) and Ehlers-Danlos Support Group (UK). Am J Med Genet 1998;77:31ŌĆō7.


48. De Coster W, Van Broeckhoven C. Newest methods for detecting structural variations. Trends Biotechnol 2019;37:973ŌĆō82.


49. Logsdon GA, Vollger MR, Eichler EE. Long-read human genome sequencing and its applications. Nat Rev Genet 2020;21:597ŌĆō614.




50. Liu Y, Chen M, Liu J, Mao A, Teng Y, Yan H, et al. Comprehensive analysis of congenital adrenal hyperplasia using long-read sequencing. Clin Chem 2022;68:927ŌĆō39.



51. Tantirukdham N, Sahakitrungruang T, Chaisiwamongkol R, Pongpanich M, Srichomthong C, Assawapitaksakul A, et al. Long-read amplicon sequencing of the CYP21A2 in 48 Thai patients with steroid 21-hydroxylase deficiency. J Clin Endocrinol Metab 2022;107:1939ŌĆō47.



52. Marino R, Ramirez P, Galeano J, Perez Garrido N, Rocco C, Ciaccio M, et al. Steroid 21-hydroxylase gene mutational spectrum in 454 Argentinean patients: genotypeŌĆōphenotype correlation in a large cohort of patients with congenital adrenal hyperplasia. Clin Endocrinol (Oxf ) 2011;75:427ŌĆō35.



53. Finkielstain GP, Chen W, Mehta SP, Fujimura FK, Hanna RM, Van Ryzin C, et al. Comprehensive genetic analysis of 182 unrelated families with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J Clin Endocrinol Metab 2011;96:E161ŌĆō72.



54. Krone N, Braun A, Roscher AA, Knorr D, Schwarz HP. Predicting phenotype in steroid 21-hydroxylase deficiency? Comprehensive genotyping in 155 unrelated, well defined patients from Southern Germany. J Clin Endocrinol Metab 2000;85:1059ŌĆō65.



55. Wang R, Yu Y, Ye J, Han L, Qiu W, Zhang H, et al. 21-hydroxylase deficiency-induced congenital adrenal hyperplasia in 230 Chinese patients: Genotype-phenotype correlation and identification of nine novel mutations. Steroids 2016;108:47ŌĆō55.


56. Koyama S, Toyoura T, Saisho S, Shimozawa K, Yata J. Genetic analysis of Japanese patients with 21-hydroxylase deficiency: identification of a patient with a new mutation of a homozygous deletion of adenine at codon 246 and patients without demonstrable mutations within the structural gene for CYP21. J Clin Endocrinol Metab 2002;87:2668ŌĆō73.


- TOOLS
- Related articles in APEM