COVID-19 and diabetes in children
Article information
Abstract
This review describes the impact of coronavirus disease 2019 (COVID-19) in children and adolescents, investigating changes in diabetes presentation during the COVID-19 pandemic, possible links between severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) infection and diabetes, and mechanisms of pancreatic β-cell destruction. Although glycemic control in individuals with already known diabetes mellitus did not worsen during the pandemic, there was a worrying increase in diabetic ketoacidosis in children with new-onset diabetes, probably due to containment measures and delayed access to emergency departments. Moreover, new evidence suggests that SARS-CoV-2 has the capacity to directly and indirectly induce pancreatic β-cell destruction, and the risk of newly diagnosed diabetes after COVID-19 increased in both children and adults. While long-term studies continue to follow children with SARS-CoV-2 infection, this review discusses available findings on the relationship between COVID-19 and diabetes. It is important to emphasize the need to maintain close links between families of children with chronic conditions and their pediatricians, as well as to promote early access to healthcare services, in order to reduce dangerous delays in diabetes diagnosis and prevent diabetic ketoacidosis.
Highlights
· A link between COVID-19 and new diabetes onset was found. Moreover, there was an increase in diabetic ketoacidosis in children with new-onset diabetes. It is important to elaborate new strategies to avoid delays in diabetes diagnosis and to prevent diabetic ketoacidosis.
Introduction
In December 2019, a new infectious disease was observed in Wuhan, Hubei Province, China, where many cases of unknown-origin pneumonia were reported. The disease, subsequently identified as coronavirus disease 2019 (COVID-19), spread rapidly in China and then across the world. Mainly presenting as a respiratory illness, in older patients or people suffering from underlying conditions COVID-19 is more likely to result in acute respiratory distress syndrome (ARDS), multiorgan failure (MOF), and even death [1]. Long-term sequelae, commonly known as "long COVID," may also be evident in the postacute phase, beyond 4 weeks from the start of symptoms [2].
This new pandemic also affects children. While general symptoms are nonspecific and similar to those caused by other common viral respiratory infections, extrapulmonary manifestations in children have been described in the literature [3].
A link to endocrine disorders was also found. Severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) demonstrated the capacity to affect multiple systems other than the respiratory system and data about the endocrine manifestations of COVID-19 eventually emerged. As an example, adrenal dysfunction has been reported and SARS-CoV-2 receptors have been identified in adrenal glands [4]. Abnormalities in thyroid function, associated with low thyroidstimulating hormone and low T3 levels, have been observed in patients with severe disease [5]. Moreover, patients already affected by endocrine disorders were found to face increased risk for severe manifestations of COVID-19.
Studies of COVID-19 cases revealed that obesity, diabetes and uncontrolled hyperglycemia increase the risks of hospitalization, intensive care unit admission, and adverse outcomes of SARS-CoV-2 infection [6-9]. COVID-19 is also associated with worsening diabetes symptoms. During the pandemic, a great number of children received delayed diabetes diagnosis. This new disease altered the presentation of diabetes itself and the severity of diabetic ketoacidosis (DKA) in both patients with known diabetes and those with new-onset disease [10].
A Centers for Disease Control (CDC) study found that children who recover from COVID-19–even children who were positive but asymptomatic–are at higher risk of developing type 1 diabetes (T1D) or type 2 diabetes (T2D) after infection [11]. This increased risk for diabetes in children who had COVID-19 underlines the need to develop adequate preventive measures as well as effective chronic disease prevention programs. It is important to emphasize the need for long-term monitoring of consequences following SARS-CoV-2 infection, including symptoms of new diabetes onset [11].
COVID-19 in children
While initial data showed that children accounted nearly for 1%–5% of diagnosed COVID-19 cases, at the time of writing, reports from the American Academy of Pediatrics suggest that children represent approximately 19% of all cases [12]. Clinical manifestations of COVID-19 cases are typically milder in children compared to ones adults, and their prognosis is better. The disease, when not asymptomatic, often presents with fever, cough, or other moderate upper respiratory tract infection symptoms. Additional manifestations can be diarrhea, vomiting, or other gastrointestinal symptoms. These findings seem to be associated with both limited exposure to the virus among children (due mainly to local lockdowns during the first wave of COVID-19) and host factors [13,14].
Considering that the virus will not stop circulating and will overlap with other contagious diseases, particularly in children, a future challenge will be to differentiate symptomatic COVID-19 in children from influenza or infections caused by other viruses and bacteria [15].
Young children, primarily infants, can be particularly vulnerable to SARS-CoV-2 infection [13] and suffer from severe and critical forms of disease. A small proportion of children develop hyperinflammation, known as Multisystem Inflammatory Syndrome in Children (MIS-C). Patients with MIS-C have a high frequency of gastrointestinal symptoms (71%), including abdominal pain and diarrhea, and have fever as a distinctive tract [16]. Cardiovascular dysfunction is the most frequently described physiological abnormality, leading to hypotension and echocardiographic irregularities. Severity of illness is generally high, often requiring admission to an intensive care unit [16].
Just as in adults, a variable percentage of children experience a broad range of symptoms 3 to 12 weeks after the documented infection. So-called "long COVID" includes symptoms as varied as chest pain, palpitations, headache, brain fog, low mood, and other mental health conditions such as depression and anxiety; in some people there may be more subjective, aspecific symptoms resembling those typical of chronic fatigue syndrome [17]. The prevalence of long COVID varies considerably across studies, due in part to the lack of a distinct definition of "long COVID" itself.
The increase in primary care consultations after COVID-19 infection in all age groups has been highlighted (including up to 6 months after infection in children aged 1–5 years). However, half of children without positive tests also experienced headache, fatigue, sleep disturbance, and lack of concentration during the pandemic [18]. It is a great challenge to distinguish symptoms of long COVID from symptoms attributable to the pandemic itself and to lockdown measures, and the fact that almost all long COVID studies lack a control group is a relevant limitation [17,19].
The only way to limit viral dissemination, specifically during the first waves of COVID-19 (when vaccines were not available), was quarantine. Almost half of the world was under a strict "lockdown"; in Europe, lockdowns began reducing viral circulation after the first 3 weeks and continued to show results as the days went by [20]. School closure led to the loss, for a considerable number of children, of access to school-based healthcare, special services, and nutrition programs. School closures also resulted in increased stress, anxiety, sadness, and depressive symptoms among children. Moreover, in the first phase of the lockdown all physical activities (both indoor and outdoor) were shut down and together with the prevalence of a more sedentary lifestyle, caused increases in children's body mass index (BMI) and augmented prevalence of obesity [21].
The CDC analyzed data from IQVIA's Ambulatory Electronic Medical Records database and found that persons aged 2–19 years with moderate or severe obesity before the pandemic faced higher rates of increase in BMI [22]. Weight gain, particularly among already overweight and obese children, produces ongoing metabolic variations that increase the risk for T2D, hypertension, and depression. The necessity of obesity prevention and management strategies both during and following COVID-19 pandemic therefore becomes clear [22,23].
It is important not only to promote efforts to fight COVID-19 through immunization and pharmacological treatments, but also to assess preventive strategies and medical care for potentially high-risk populations [24]. Close contact with healthcare professionals should be guaranteed to all children and their parents, with the help of telemedicine if necessary.
COVID-19 and DKA in children
Diabetes mellitus is one of the leading causes of morbidity and mortality in the world, reducing the life expectancy of those affected by its microvascular and macrovascular complications. The Global Burden of Disease (GBD) study found that the prevalence among subjects aged 1-19 years increased from 5.7 million in 1990 to 8.8 million in 2017. Considering all age groups, cases of diabetes have almost doubled since 1990. The GBD study also found that the increasing global burden varied by type of diabetes and region: increases in T1D have mainly affected high income regions such as Europe and the United States [25].
Due to the current COVID-19 pandemic lifestyles have changed drastically worldwide, with physical and psychological consequences. Patients with pre-existing chronic conditions such as T1D have suffered the most in this situation. The presence of a chronic condition is an important risk factor for higher rate of progression to ARDS in hospitalized patients with COVID-19 [7].
There are conflicting data regarding the incidence and the severity of presentation of diabetes during the COVID-19 pandemic. While some reports have noted a decrease in the incidence of T1D, others detected increases in the number of DKA cases and, particularly, severe DKA during the COVID-19 pandemic. DKA is an important metabolic alteration that causes hyperglycemic emergencies in patients with diabetes mellitus. Once thought to be a condition only occurring in patients with T1D, DKA can also occur in T2D, particularly when patients undergo stressful conditions such as surgery, trauma, and infections [26].
The International Society for Pediatric and Adolescent diabetes criteria classify DKA [27] as a condition that comprises:
- Blood glucose > 11 mmol/L (~200 mg/dL);
- Venous pH <7.3 or bicarbonate <15 mmol/L;
- Ketonemia and ketonuria.
DKA severity is defined as follows [27] :
- Mild DKA: venous pH <7.3 or bicarbonate <15 mmol/L;
- Moderate DKA: venous pH <7.2, bicarbonate <10 mmol/L;
- Severe DKA: venous pH <7.1, bicarbonate <5 mmol/L.
Investigations of the prevalence of diabetes and DKA during the pandemic yielded interesting but partially conflicting data. Some studies showed that children and adolescents already diagnosed with T1D did not experience deterioration of glycemic control during the current pandemic and the lockdown period. In children, glucose control actually improved during the pandemic with augmented time in range (TIR) and reductions of average blood glucose level. This improvement reflected the shift to full-time parental control of meals, glycemic levels, and insulin administration [28].
Some retrospective evaluations of glycemic control included individuals with a median age of 14.2 years and T1D in the period that occurred just prior the SARS-CoV-2 outbreak in Italy and during the first lockdown. In these adolescents, glycemic control did not worsen; it actually improved in those who regularly continued physical activity during quarantine. The improvement was attributed to the continuous presence of parents at home and to the reduction of the unpredictability of school and after-school activities [29]. The pandemic, therefore, has not led to a significant worsening in disease control in children who already had diagnoses of diabetes.
Regarding new diabetes diagnoses, in particular T1D, another issue has to be addressed. During the pandemic, there have been significant reductions in pediatric emergency visits. In the United States (US), before 2020, pediatric admissions overall displayed winter predominance, associated with an increase in infectious respiratory conditions. However, during 2020, there was a dramatic decrease in pediatric admissions to emergency departments. A maximum reduction of 45.5% in pediatric admission rates for non–COVID-19–related diagnoses was detected in 2020 with significant reductions in all examined diagnoses except for birth [30]. One explanation of this finding could be fear of contracting COVID-19 and reductions in seasonal infectious diseases due to lockdown and self-isolation [31,32]. The pandemic situation directly and indirectly forced patients with illnesses other than COVID-19 to stay at home. Similar findings have been described in other countries.
T1D is one of the most common chronic diseases in children and its signs and symptoms usually develop quickly. Physicians and parents are pivotal in the prevention of DKA. The key question in this context was about the frequency of DKA during the pandemic. During the COVID-19 pandemic, higher incidences of severe and moderate DKA at T1D initial presentation have indeed been reported (Table 1).

Summary of reviewed studies about the impact of COVID-19 pandemic on diabetes presentation at diagnosis
Initial studies examined the early phase of the pandemic, during which local lockdowns were imposed. In Italy, a cross-sectional study compared data from 53 of 68 centers (77.9%) belonging to the Italian Society for Pediatric Endocrinology and Diabetes (SIEDP/ISPED). Two periods were studied, the first between 20 February and 14 April 2019, and the second between 20 February and 14 April 2020. While there were fewer diabetes cases during the pandemic, the number of DKA and, particularly, of severe DKA cases increased significantly [10].
In a prospective multicenter study, data during the first wave from March to December 2020 from all pediatric diabetes centers in Lombardy, Italy, were examined. The observation period was extended to the next few months during which the restrictions of the first wave were partially eased. They found higher proportions of both DKA and severe DKA (over total DKA diagnoses) during the first wave, probably due to delayed access to Emergency Departments and higher numbers of DKA diagnoses over total T1D diagnoses even during the second wave of the pandemic [33].
These results, showing an increase in the prevalence and severity of DKA in new-onset diabetes, are consistent with reports from other countries. Significant increases in DKA and severe DKA at diabetes diagnosis were reported during the COVID-19 pandemic among children and adolescents in Germany. These findings were attributed to underlying causes such as fear of accessing the health care system and reduced medical services during the lockdown [34]. A Polish study compared 2 groups, from 2019 and 2020. Overall, they observed increased DKA incidence (52% vs. 49% of newly diagnosed T1D), with a further increase in severe DKA in 2020 versus 2019. This increased prevalence of severe DKA was attributed to a combination of factors: lockdown and difficulty contacting health care professionals for non-COVID-19-related diseases, parental unawareness of classic diabetes symptoms, and shorter duration of symptoms in children with DKA [35].
In the United Kingdom, data were collected from several diabetes units (76 from England, 10 from Wales, and 2 from Northern Ireland). The number of children with DKA at diagnosis was higher than in previous reports. It was also estimated that 20% of newly diagnosed T1D cases were detected after a relevant delay related to fear of contracting COVID-19 or to inability to reach healthcare services [32].
An increase in glycosylated hemoglobin among new-onset T1D during the pandemic was reported, along with an 11% increase in DKA diagnoses, in a study of Saudi children. Data from the first year of the pandemic were compared to data from the same period of the previous year [36]. Other studies examined data (for both children and adults) from 7 large US medical centers, finding that DKA frequency increased among T1D patients during the pandemic. During the first (March–May 2020) and second (August–October 2020) COVID-19 waves, higher proportions of patients had DKA compared to the same periods in the previous year (first wave: 7.1% vs. 5.4%, P<0.001; second wave: 6.6% vs. 5.7%, P=0.001) [37]. In another study of 615 children admitted with new-onset diabetes in a tertiary care children’s hospital between March 2018 and December 2020, children admitted to the hospital with new-onset diabetes in the pandemic and post-pandemic period were more likely to present with DKA compared to those in the pre-pandemic period [38].
Interestingly, American studies highlight a significant association between ethnicity and DKA proportion, with more DKA diagnoses in nonwhite children (Black, American Indian and Alaskan Native patients) [37,38]. Increase in DKA severity in T2D was also described during the pandemic among nonwhite children (Black or African America, Hispanic, Latino). These adolescents had worse glycemic and metabolic control overall even before the pandemic, mostly as a consequence of socioeconomic status factors and higher BMI [39].
Such concerning increases in DKA and, specifically severe DKA, must be considered major impacts of COVID-19 pandemic on healthcare systems. In most countries medical resources were shifted towards pandemic management, and telephone contact became the only way to seek medical consultation for other conditions. Patients were recommended to stay at home [40], where parents were often unaware of potentially alarming diabetes symptoms [10] such as the onset of polyuria, nycturia, weight loss, fatigue, and polydipsia. However, these are not the only possible explanations: a distinction must be drawn between children who tested positive for SARSCoV-2 infection and children who did not or for whom tests were not performed.
It is now recognized that diabetes affects the prognosis of COVID-19. Studies have found that a pre-existing diabetes diagnosis did not increase the risk of contracting COVID-19, but diabetes can worsen the outcomes of disease [41]. Poor glycemic control is associated with worse COVID-19 prognosis while improved glycemic control is linked with better outcomes [42]. Another question arises from new reports that seem to demonstrate a link between newly diagnosed diabetes in patients with prior COVID-19 infection. In adults, an increased risk of diabetes was reported as a postacute, long-term consequence of SARS-CoV-2 infection [43]. It remains to be seen whether this condition will persist or be reversible with time.
In children, COVID-19 might lead to diabetes through direct and indirect effects of infection on endocrine and exocrine pancreatic cells and through the hyperinflammation that it promotes, and may lead to ketosis and ketoacidosis [44,45]. In a recent study of newly diagnosed diabetes in children who tested positive for SARS-CoV-2, data from 2 major US medical health insurance claims databases indicated that new diabetes diagnoses were more likely to occur in patients who had COVID-19 in the previous 30 days than those who did not [11]. These results were also traced back to the effects that SARS-CoV-2 infection may have on organs involved in diabetes risk. Moreover, infection helps quicken the transition from prediabetes to diabetes. A limitation of that study was that some patients with highly likely SARS-CoV-2 infections did not have documented positive tests, and might have been misclassified as not having COVID-19 [11]. On the other hand, some studies were conducted without including homogeneous information about SARS-CoV-2 testing status or about the presence or absence of serum antibodies [33,38,46]. This is clearly a source of bias that may lead to inaccurate classification of some patients and result in underestimations of disease effects.
COVID-19 and pathogenetic mechanisms of β-cell destruction
RNA and DNA viruses are known to be potential causal agents for diabetes in people prone to diabetes due to genetic and/or physiologic factors. Multiple pathogenetic mechanisms of β-cell damage have been proposed in animal and human models of diabetes. Some viruses have a tropism for β cells and the infection has the capacity to induce direct cellular lysis. In other cases, the infection promotes inflammation, dedifferentiation [47] of β cells, or turns the infected cell into a target for the immune system [48,49].
The onset of T1D and, in some cases, of fulminant T1D, has been reported during and after acute infection with mumps virus [50], rotavirus [51], enteroviruses, particularly coxsackie virus [52], and many others. Regarding rotavirus infection, with the diffusion of live attenuated rotavirus vaccination a new question arose: could the vaccination itself be associated with T1D? While a triggering connection between some wild-type rotavirus infections and diabetes has been confirmed [51], recent studies found that children exposed to the vaccine did not have a higher risk of developing T1D compared to children who did not receive vaccination against rotavirus [53]. In addition, some studies showed a reduction of prevalence of T1D after the vaccination. In a cohort study of 1,474,535 infants in the US from 2001–2017, there was a 33% reduction in the risk of type 1 diabetes with completion of the rotavirus vaccine series compared to unvaccinated children [54]. Diabetes is not only an independent risk factor for admissions into intensive care unit and related to worse outcomes in patients with COVID-19 [7-9], but SARS-CoV-2 infection is also thought to be a potential causal factor for diabetes.
Coronaviruses are a large group of viruses that belong to Family Coronaviridae, Order Nidovirales. They have been further categorized into 4 strains: α, β, γ, and δ coronaviruses. Like all viruses in Nidovirales, SARS-CoV-2, which belongs to the β-coronaviruses and infects mammals, is an enveloped virus with a single strand, nonsegmented positive-sense RNA genome [55]. It contains 4 structural proteins: spike (S), envelope (E), membrane (M), and nucleocapsid (N). Although not entirely the same, SARS-CoV-2 shares about 82% of its entire sequence with SARS-CoV and MERS-CoV and more than >90% sequence identity for essential enzymes and structural proteins [56].
The entry of viral particles into host cells is mediated by the S glycoprotein that binds to the receptor on host cells [57]. The S protein is structured to form homotrimers that bulge from the viral membrane, giving it the typical crown-like appearance [58].
In SARS-CoV-2 S the trimeric S protein binds to the cellular receptor angiotensin-converting enzyme 2 (ACE2), mediating the fusion of the 2 membranes, viral and cellular [59]. The virus is then primed by proteases; specifically, S protein is cleaved by a host cell furin-like protease into 2 separate subunits, S1 and S2 [55,60]. While furin processes the S1-S2 site, TMPRSS2, a host type II transmembrane serine protease, intervenes in cleavage at the S2 site. Both proteases are fundamental and cannot compensate for each other [61]. Another entry pathway proposed for SARS-CoV-2, the so-called "late" pathway (whereas the "early" one is mediated by furin-like proteases and TMPRSS2), is based on cathepsin, a lysosomal protease that mediates cleavage at the S2 site in the late endolysosome. Cathepsin L, in particular, plays a role in SARS-CoV and SARS-CoV-2 entry [58].
Knowing where ACE2 is expressed supports understanding about SARS-CoV-2 tropism and the mechanisms of susceptibility for SARS-CoV-2 infection. The ACE2 receptor is a carboxypeptidase consisting of 805 amino acids and shares homology with ACE. There are multiple studies of different ACE2 expression levels in airways. It was detected mostly in ciliated airway epithelial cells, suggesting that the infection tends to involve mainly the proximal airways.[62,63].
The protein expression of ACE2 has been observed in various human cell types, tissues and organs (enterocytes, renal tubules, gallbladder, cardiomyocytes, male reproductive cells, placental trophoblasts, ductal cells, eye, and vasculature) [64]. Previous studies suggest that ACE2 is also expressed in pancreatic tissue. Some authors, through the use of multiple reagents and antibodies, showed that ACE2 is expressed in human pancreatic islets and is preferentially expressed in insulin-producing β cells [65]. However, there are other immunohistochemistry studies in which minimal to no expression of ACE2 was detected [66,67], thus decreasing the chance of a direct SARS-CoV-2 infection of β cells through ACE2. Some authors found high expression of ACE2 in pancreatic pericytes and in acinar and islet microvasculature, with no evidence of ACE2 expression by α or β cells. On the other hand, several studies, all conducted using immunohistochemistry, support the expression of ACE2 in pancreatic islets [65,68,69]. The discrepancy may be explained by differences in antibody sensitivity, immunodetection method sensitivity, and tissue preparation methods [65].
As for studies in which ACE2 presence in pancreatic β cells was detected, the expression of the receptor was only observed using 2 out of 3 antibodies tested, with ACE2 islet signal detected using MAB933 and Ab15348 antibodies, while Ab108252 antibody did not show positivity within the islet parenchyma. The explanation provided was that the prevalent ACE2 isoform expressed in the islet was the short one. ACE2, as it has been recently documented, has a 459 amino acid short isoform that can be coexpressed together with the full-length form, which is of 805 amino acids. However, the functional role of this short-length isoform has not been established, although it can potentially interact with the full-length form or with membrane proteins, thus modulating SARS-CoV-2 susceptibility [65].
Other evidence supporting direct pancreatic infection mediated by SARS-CoV-2 is provided by several studies. ACE2 mRNA and protein were found in both endocrine and ductal pancreatic cells. Moreover, upon examining entire pancreatic sections stained with hematoxylin-eosin multiple thrombotic areas were found in SARS-CoV-2 infected subjects, alongside higher levels of pancreatic lipase. In these subjects higher levels (if compared with non-infected controls) of intercellular adhesion molecule 1, ICAM1, a marker of endothelial inflammation and dysfunction, were also found [70].
With regard to mechanisms of pancreatic β-cell damage, viruses may have the potential to activate β-cell intracellular signaling, inducing altered expression of self-antigens on the surface of the cell, thus inducing a process that ends in apoptosis and insulitis [71] (Fig. 1). β-cell destruction may also result after the induction of direct cellular lysis due to a massive pro-inflammatory cytokine response [72]. After the outbreak of the COVID-19 pandemic, the focus has inevitably shifted to potential mechanisms of pancreatic damage directly caused by SARS-CoV-2 infection. Even though there are conflicting data about islet and β-cell expression of markers essential for viral entry, it has been suggested that COVID-19 leads to β-cell destruction also through an indirect effect, damaging the surrounding pancreatic tissue. In some studies conducted using human autopsy tissue, it was found that pancreatic islets of SARS-CoV-2-infected subjects expressed elevated levels of pseudokinase mixed lineage kinase domain-like protein (pMLKL), a hallmark of necroptosis [68].
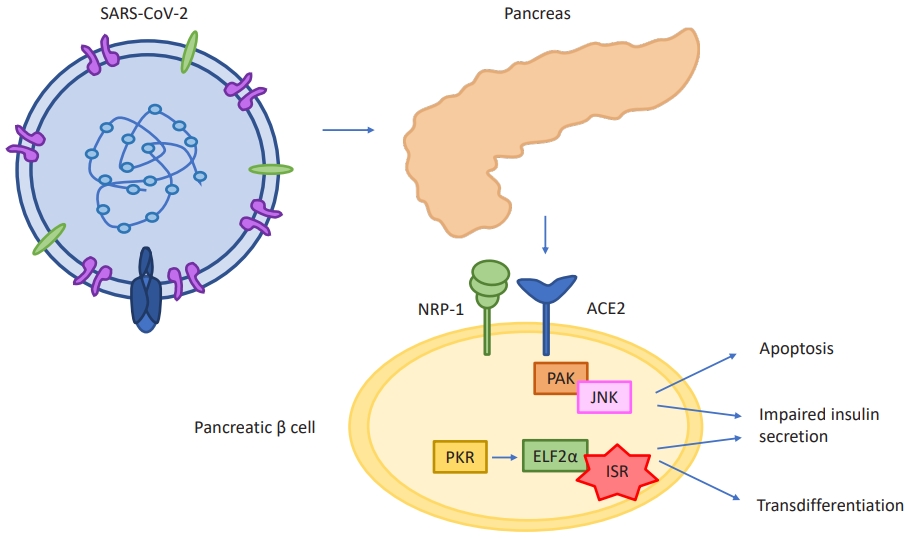
RS-CoV-2 enters the pancreatic β cell and activates β-cell intracellular signaling, inducing processes that end in apoptosis, insulitis, and transdifferentiation into α cells and acinar cells. SARS-CoV-2, severe acute respiratory syndrome-coronavirus-2; ACE2, angiotensin converting enzyme 2; NRP-1, neuropilin-1; PAK, p21-activated kinase; JNK, c-Jun N-terminal kinases; PKR, protein kinase R; ELF2α, eukaryotic translation initiation factor 2 subunit 1; ISR, integrated stress response.
Other authors determined that SARS-CoV-2 infection is associated with morphological and functional changes, including reduced insulin-secretory granules, resulting in reduced insulin content and glucose-stimulated insulin secretion. This dysfunction, alongside with cellular death, causes new-onset diabetes or worsening of pre-existing diabetes [44,73].
Upon infecting primary human islets ex vivo with SARS-CoV-2, some authors observed that the infection resulted in higher chemokine and cytokine expression (CCL2, CXCL1, CXCL2, CCL3, CCL4, CXCL5, CCL8, IL1RN, IL1B). In addition, a β-cell transdifferentiation path was identified: the infection caused a reduction of insulin content and secretion along with a reduction in protein processing and expression [74]. Creating this hyperinflammatory state, COVID-19 may easily induce ketosis and ketoacidosis [57,75]. Case reports and studies, lastly, described the development of necrotic and non-necrotic pancreatitis, and elevations in lipase and amylase during and following the infection [67,76].
To sum up, while not all cases result in the onset of new diabetes, cellular destruction mechanisms that develop during SARS-CoV-2 viral infection contribute to impaired glucosestimulated insulin secretion and global metabolic dysregulation in patients with COVID-19, often worsening the course of the infection.
COVID-19, obesity, and T2D
The prevalence of overweight and obesity is rising worldwide. According to the World Health Organization, in Europe, America, and the Eastern Mediterranean approximately half of the population is overweight or obese. Lower prevalences of obesity are reported in Africa and Asia but with a rising trend [77]. The association between elevated BMI and adverse health consequences is known not only in adults, but also in children and adolescents. Strict connections between abdominal obesity and the incidence of prediabetes, T2D, hypertension, dyslipidemia, nonalcoholic fatty liver disease (NAFLD), and metabolic syndrome have been documented [78-80].
During the COVID-19 pandemic, a study of 432,302 children (ages 2–19 years) found that the rate of BMI increases nearly doubled compared to the pre-pandemic period [22]. This result, consistent with those of other studies, highlights the importance of obesity prevention and management strategies during and after the pandemic period.
In 2020, China was the first country to implement a strict national lockdown to combat the emergence of SARS-CoV-2. This measure was quickly embraced by Italy and, afterwards, by a large number of countries worldwide. At a time when the world was facing a new and unfamiliar disease, vaccines and specific pharmacological treatments were not available and health resources had to be targeted and rationalized, lockdown prove to be an effective measure for virus containment [20,81]. But not without a cost: the unexpected disease outbreak threatened the mental health of both affected and non-affected people [82]. The lockdown had a strong impact on adults, and the burden of this situation on children and adolescents should not be underestimated. Physical and psychological changes were reported in both children and adults especially during the first lockdown, with stricter measures. The main effect was a drastic change in feelings and emotional state. The major reported condition in children was mood instability, often associated with anxiety and with a sensation of fear of getting/of relatives getting COVID-19. Lifestyle changes occurred, with sleep disorders becoming predominant. Increases in screen time were observed, as well as reductions of both indoor and outdoor physical activities [83]. Hence, despite being an effective method as for virus containment, lockdown showed a series of significant negative effects.
In parallel with psychological issues, the COVID-19 pandemic also exacerbated the issue of childhood overweight and obesity. Lockdown restricted all kinds of daily activities with negative changes in eating and sleep behaviors and reductions in physical activity in children and adolescents. These changes have direct effects on body weight, with children who reported decrease in physical activity and increase gaining more body weight [84,85]. The effects were greater in already overweight and obese children who experienced major increases in BMI with a greater risk of metabolic and biochemical changes [85-87].
Questionnaires and telephonic interviews were administered during and after lockdown to gather information about lifestyle, diet, sleep behavior, physical activities, and mental health during the weeks of mandatory home confinement. In a crosssectional study carried out by the pediatric endocrinology department of a tertiary university hospital in Rome, pediatric patients with already elevated BMI had worse diets and greater increase in BMI during lockdown, perhaps due to reductions in the consumption of fresh food, especially fruit and vegetables, associated with higher intake of carbohydrates and junk food during a period in which sleep and activity behaviors also changed dramatically [88]. Comparable results were showed in a longitudinal study, also conducted in Italy, in which adverse variations in eating and activity behaviors were more noticeable in obese children and adolescents [85].
In response to factors both related to the infection and the lockdown, many studies also reported an increase in T2D incidence. In a retrospective study, the frequency of T2D cases grew sharply after the onset of the COVID-19 pandemic. Children with new-onset T2D had a median age at admission of 11.3 years. In particular, the relative frequency of T2D increased significantly in the cohort of children with DKA, and the percentage of moderate DKA was higher among children with T2D than in T1D [38]. Comparable results about T2D and DKA were observed at Children's Hospital, Los Angeles, where the prevalence of DKA increased significantly from <10% to 20% from 2018-2019 to 2020 [89]. Other retrospective studies reported an increased incidence of T2D compared to pre-pandemic years mostly as an indirect effect of the pandemic [90,91]. Interestingly, cases increased among non-Hispanic Black males, mostly due to social and economic disparities that predated the pandemic [91,92]. Lockdown also resulted in quick worsening in glycemic control in patients with already known T2D, unlike children with T1D, who experienced improvement in glycemic values during home confinement [93].
Attention should be focused on the increased incidence of T2D in children and adolescents after lockdown. Most likely, the significant changes in children’s habits during lockdown enhanced the risk of obesity, and the increase of BMI was found to be higher among already overweight and obese children. [84-86] The growth in obesity rate led, at the same time, to an increase in the incidence of T2D [90,91,94].
In the broader perspective, families should be supported and adequate measures should be implemented to help children and young people maintain adequate activity levels together with healthy sleep routines and eating habits, even in a pandemic situation. Strategies to increase awareness about childhood obesity, diabetes, and their management should be implemented, in order to help parents get in touch with healthcare professionals so that children can be diagnosed early [90,95,96]. At the same time, in patients with diagnosed T2D, physicians should be aware of likely changes in dietary habits and in physical activities triggered by the pandemic context. The impact of these changes on glycemic control and on glycemic TIR should be considered and the dosages of antidiabetic drugs should be adjusted, if necessary, to maintain control of the disease without enhancing the risk of complications.
Conclusions
The COVID-19 pandemic has raised several challenges for healthcare professionals globally, as well as for patients. Studies in adults that have shown how patients with diabetes are more predisposed to a severe course of COVID-19 [97]. Other data suggest that SARS-CoV-2 infection may result in metabolic dysregulation even in subjects without a history of diabetes, and the infection may not only worsen pre-existing diabetes, but also cause new diabetes onset [98].
For children, current data are considerably more limited. Although clinical manifestations of COVID-19 in children are typically milder in comparison to adults, endocrine imbalances may appear in children too. During the pandemic, although T1D diagnoses did not increase, more children with new-onset T1D presented with DKA and severe DKA at diagnosis. The increase in DKA at diagnosis most likely resulted from delays in diagnosis due to difficultly accessing healthcare during the pandemic and lack of parental recognition of common symptoms of diabetes [99].
Reductions in emergency department access have been reported in most countries, mainly during the first, strictest lockdown [30-32]. The fear of contracting COVID-19 and advice given by healthcare workers not to visit emergency departments have been suggested to explain the advanced clinical presentation of diabetes in children and adults [100]. However, the pandemic did not lead to worsening of disease control in children with already diagnosed T1D, who probably benefitted from the fact that, with their parents at home, their blood glucose levels were monitored more strictly. Moreover, fluctuations attributable to less proper nutrition and to the unpredictability of outdoor activities (school, physical activity) fell short [29]. In addition to the increase of DKA at T1D diagnosis, COVID-19 indirectly raised T2D risk in children and adolescents through increases in body weight and BMI that can be traced back to the effects of the pandemic and mandatory home confinement [38,88,90].
In patients with documented SARS-CoV-2 infection, associations between COVID-19 and diabetes were found not only for adults, but also for children. COVID-19 might lead to diabetes through direct attack of pancreatic β cells and, indirectly, through cytokine storm [11,98]. To combat this inauspicious trend, a number of precautions may be taken. Pediatricians and healthcare workers in general should point out to parents that, even in a pandemic context, the risks of avoiding emergency departments and hospital should be considered alongside the risk of contracting COVID-19. This concept should be stressed using the right communication strategies in order to contain the dangers caused by delayed or missed emergency healthcare recourse during a pandemic [101].
In order to highlight potential symptoms that should warn parents that their children need care, new campaigns for diabetes and DKA prevention could be effective. An example is the massive campaign for DKA prevention fostered in Parma in the period between 1991 and 1997, which retained effectiveness even 8 years after its initial promotion [102]. Periodically renovating these informational campaigns can be a useful strategy to warn parents, emphasizing the necessity to seek medical help and thus shortening the latency period between the first symptoms and the diagnosis.
In the current pandemic, children with chronic conditions such as diabetes need uninterrupted access to medical care, through telemedicine if necessary, and should be monitored strictly both by parents and pediatricians so that they maintain healthy nutrition, continue regular physical activity, and continue their glucose monitoring even when confined at home. The ISPED has proposed some practical recommendations for pediatric patients with diabetes during COVID-19 pandemic [103].
Appropriate treatment adjustments should be made in individuals already having diabetes and in newly diagnosed patients. While long-term follow-up studies will further elucidate the association between COVID-19 and diabetes, in clinical practice it is vital to extend the follow-up period of these children. Clinicians should consider new-onset diabetes as a possible consequence of the metabolic imbalance caused by SARS-CoV-2 infection.
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Funding
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author contribution
Conceptualization: SP; FC; Data curation: SP; FC; Project administration: SP; FC; Visualization: SP; FC; Writing - original draft: SP; Writing - review & editing: SP; FC
