 |
 |
- Search
| Ann Pediatr Endocrinol Metab > Volume 28(3); 2023 > Article |
|
Abstract
Purpose
Overweight (OW)/obese girls tend to have an earlier pubertal onset than girls with normal weight. However, only a few studies have reported the progression of puberty in these girls. This study aimed to identify risk factors for rapid pubertal progression in OW/obese girls presenting with precocious breast development.
Methods
This retrospective cohort study reviewed the medical records of 110 OW (body mass index [BMI] Ōēź85th percentile for age and sex) and 213 nonoverweight (NW, BMI <85th percentile for age and sex) girls who presented with breast budding before 8 years of age. OW girls were divided into 2 subgroups: girls with central puberty progression before 9 years of age (OW-RP) and those without (OW-SP).
Results
Progression to central puberty before the age of 9 was more common in NW girls than in OW girls (83.8 % vs. 65.2 % in NW vs. OW group, p<0.001), and progression-free survival for 1, 2, and 3 years was higher in the OW group (p<0.001). In a subgroup analysis of OW girls, the OW-RP subgroup had more advanced bone age (BA) at the first visit (p=0.047) and higher initial luteinizing hormone (LH, p=0.010) levels than the OW-SP subgroup. Being NW (p=0.001) and having more advanced BA (p=0.023) at the initial workup were the risk factors for pubertal progression before age 9.
┬Ę Pubertal progression seems to be slower in overweight (OW) girls than in nonoverweight girls presenting with precocious breast development. However, it can progress rapidly in OW girls with particularly pronounced bone age advancement and high luteinizing hormone levels at the initial workup.
With the increasing prevalence of childhood obesity, studies have shown that overweight (OW) girls tend to have earlier pubertal timing than normal-weight girls [1-3]. A study performed in Egyptian children compared girls with body mass index (BMI) Ōēź 85th percentile and < 85th percentile in terms of their mean age at breast development. Puberty started earlier in girls with BMI Ōēź 85th percentile, and excess weight was related to earlier breast development [4]. A prospective cohort study from the United States reported that girls with greater BMI reached breast stage 2 at younger ages than those with lower BMI and confirmed a trend of thelarche onset at younger ages, which is consistent with temporal changes in BMI [5]. A crosssectional study from Brazil also showed that increased BMI was associated with earlier self-assessed pubertal onset [6].
However, a meta-analysis reported no difference in the age at menarche between obese and nonobese girls [7], which suggests that the progression of puberty is slower in obese girls. Furthermore, the precise mechanisms and associated factors contributing to the progression of precocious puberty in OW or obese girls remain to be elucidated. In this study, we aimed (1) to determine whether the progression of puberty is slow in OW girls with precocious breast development, (2) to compare the characteristics of OW vs. nonoverweight (NW) girls with precocious breast development, (3) to discover the characteristics of OW girls with slow pubertal progression, and (4) to define the predictors of rapid pubertal progression in girls with precocious breast development.
This retrospective cohort study enrolled 562 girls who visited Bundang CHA Hospital for breast budding before 8 years of age, were followed up, and underwent laboratory tests, including luteinizing hormone (LH) measurements, between January 2016 and April 2020. We excluded those without an LH releasing hormone (LHRH) test (n=189) and those without sufficient data (n=31). We also excluded 19 girls (13 with increased BMI and 6 with decreased BMI) whose BMI percentile changed across the 85th percentile during follow-up because they were not considered suitable for the comparison between NW and OW girls. In that way, 323 girls who underwent blood tests at least twice, including the LHRH test at least once, were enrolled, and their medical records were reviewed (Fig. 1).
The OW group was defined as girls with a BMIŌēź85th percentile, and the NW group was defined as girls with a BMI < 85th percentile for age and sex, based on the 2017 Korean Children and Adolescent National Growth Charts [8]. For the subgroup analysis, the OW group was divided into those with progression of central puberty before 9 years of age (OW-RP subgroup) and those without (OW-SP subgroup).
Clinical and anthropometric data were reviewed: chronological age (CA), bone age (BA), bone age advancement (BA-CA), height, weight, and BMI. Laboratory results were also reviewed: LH, follicle-stimulating hormone (FSH), estradiol (E2), and dehydroepiandrosterone sulfate (DHEA-S) levels.
Height (cm) and weight (kg) were measured to the nearest 0.1 cm and 0.1 kg using a wall-mounted stadiometer (Harpenden Portable Stadiometer, Seritex, Howell, NJ, USA) and a digital floor scale (HE-24, CAS, Yangju, Korea), respectively. Pubertal development was assessed using Tanner staging [9], and BA was evaluated using the Greulich and Pyle atlas.
Serum LH and FSH levels (Atellica, Simens, Germany) and serum E2 and DHEA-S levels (Roche Cobas 8000 e801, Roche Diagnostics, Mannheim, Germany) were measured using electrochemiluminescence immunoassays. Analytical sensitivity was 0.07 U/L for LH, 0.3 U/L for FSH, 5.0 pg/mL for E2, and 0.1 ╬╝g/dL for DHEA-S.
The LHRH test was performed when clinical suspicion of rapid pubertal progression was present before the age of 9; however, it was also performed in 12 girls who were 9 years or older when their doctors had a strong suspicion of rapid pubertal progression and the girls were experiencing psychosocial stress from anxiety about the probability of early menarche. For the LHRH test, LH and FSH levels were measured at baseline and at 30, 45, 60, and 90 minutes after an IV administration of gonadotropin releasing hormone 0.1 mg [10]. In the remaining 18 children, pubertal progression was confirmed by basal LH levels. Among girls with progression to central puberty after the age of 9, the CA at confirmation of central puberty was 9.4┬▒0.2 years in the NW group (n=18) and 9.7┬▒0.3 years in the OW group (n=12) (P=0.008).
Progression to central puberty was defined as an LH peak of Ōēź5 U/L in the LHRH test (n=170 in NW group and n=61 in OW group) [11-13] or a basal LH level of Ōēź0.3 U/L (n=8 in NW group and n=11 in OW group) in girls with progressive breast development [14-17].
Of the 323 girls, 123 (57.7%) in the NW group and 72 (65.5%) in the OW group (P=0.180) underwent a laboratory workup using basal blood samples drawn before 10:00 AM.
Data are presented as the mean┬▒standard deviation. Statistical analyses were performed using IBM SPSS Statistics ver. 26.0 (IBM Co., Armonk, NY, USA). Student t-test was used to compare the following variables between groups: age, BA, BA-CA, height, weight, BMI, and hormone levels. A logistic regression analysis was used to identify the predictors of rapid pubertal progression. To compare the progression rate to central puberty before 9 years of age between the groups, the chi-squared test and a Kaplan-Meier curve analysis were used. Statistical significance was set at P<0.05.
This study was conducted in compliance with the principles of the Declaration of Helsinki and was approved by the Institutional Review Board of CHA Bundang Medical Center (2021-11-004).
Among the NW group (n=213), progression to central puberty was confirmed before 9 years of age in 160 girls and after 9 years of age in 18 girls. Thirty-five NW girls, including 22 girls younger than 9 years of age at their last visit, did not enter central puberty during follow-up. In the OW group (n=110), 60 girls entered central puberty before age 9, and 12 girls entered it after age 9. Thirty-eight OW girls, including 18 girls younger than 9 years of age at their last visit, did not enter central puberty during follow-up (Fig. 1).
Among those who had been followed until age 9 or older, 83.8% (160 of 191) of NW girls and 65.2% (60 of 92) of OW girls showed central puberty progression before 9 years of age, suggesting that progression to central puberty before 9 years of age was more common in the NW group (P<0.001). The duration of follow-up, counted from the initial visit to confirmation of central puberty progression or the last visit, was longer in OW group (20.4┬▒15.1 and 15.6┬▒11.2 months in OW and NW girls, respectively, P=0.007).
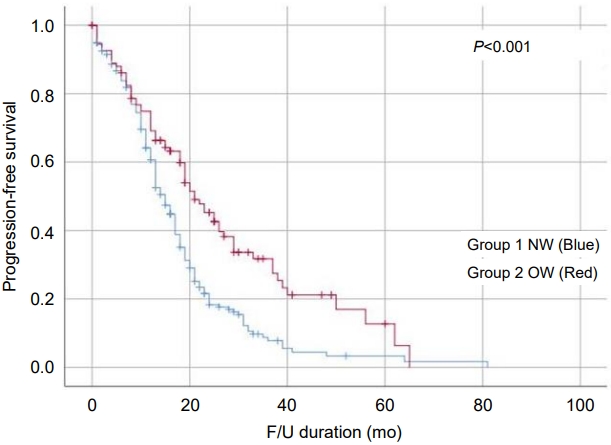
The Kaplan-Meier curve also showed slower progression to central puberty in the OW group than the NW group. Progression-free survival rates, defined as the rate without progression to central puberty among those who were followed up for 1 year (OW: 69.2% vs. NW: 60.6%), 2 years (OW: 45.3% vs. NW: 18.3%) and 3 years (OW: 31.7% vs. NW: 7.8%), were higher in the OW group than the NW group (P<0.001) (Fig. 2).
Age at the initial visit was younger (OW: 6.9┬▒0.8 years vs. NW: 7.2┬▒0.8 years, P=0.009) and BA advancement was more pronounced (OW: 16.7┬▒8.1 months vs. NW: 12.7┬▒7.5 months, P<0.001) in the OW girls than the NW girls. The initial FSH levels were significantly lower in the OW group (2.62┬▒1.7 U/L) than the NW group (3.10┬▒1.8 U/L, P=0.024), but the initial LH levels did not differ significantly between the groups (Table 1).
When progression to central puberty was first confirmed, CA did not differ between the groups, but the OW girls were taller (height standard deviation score: 1.2±0.9 and 0.7±1.0 in OW and NW girls, respectively, P=0.001), and their BA was older (OW: 10.0±1.1 years vs. NW: 9.7±0.8 years, P=0.042) and more advanced (20.6±8.1 and 15.3±7.7 in OW and NW girls, respectively, P<0.001) than NW girls. On the other hand, ΔBA, ΔBA-CA, plasma gonadotropin, and estradiol levels did not differ statistically between the groups, although the DHEA-S level was significantly higher in the OW group than the NW group (85.0±39.3 μg/dL vs. 62.7±30.8 μg/dL in OW and NW girls, respectively, P=0.011) (Table 1).
In the subgroup analysis of OW girls, the OW-RP subgroup had more advanced BA at the first visit (OW-RP: 17.7±7.3 vs. OW-SP: 14.4±7.8, P=0.047) and higher initial LH (OW-RP: 0.26±0.7 vs. OW-SP: 0.02±0.1 U/L, P=0.010), FSH (OW-RP: 3.17±1.7 vs. OW-SP: 1.84±1.3 U/L, P<0.001), and E2 (OW-RP: 11.3±12.2 vs. OW-SP: 4.5±6.4 pg/mL, P=0.001) levels than the OW-SP subgroup. When progression to central puberty was first confirmed, the BA was significantly older in the OW-SP subgroup (OW-SP: 11.2±0.9 vs. OW-RP: 9.8±1.0 years, P<0.001). Although ΔCA was much longer in the OW-SP subgroup, BA-CA and ΔBA-CA did not differ significantly between the subgroups (Table 2).
A logistic regression analysis was performed to identify the predictors for rapid pubertal progression, and it showed that being OW was unlikely to be related to the risk of pubertal progression before 9 years of age (OR [95% confidence interval {CI}], 0.313 [0.157ŌĆō0.624]; P=0.001). Initial BA advancement (OR [95% CI], 1.056 [1.007ŌĆō1.107]; P=0.023) and higher FSH (OR [95% CI], 1.432 [1.125ŌĆō1.822]; P=0.003) and E2 levels (OR [95% CI], 1.062 [1.015ŌĆō1.112]; P=0.009) were the risk factors for the progression of central puberty before age 9 (Table 3).
It has been reported that OW children experience earlier onset of thelarche than those with normal weight, and high leptin levels in OW children might play an important role in gonadotropin secretion and earlier pubertal onset [7,11,18]. Although our study did not examine the prevalence of precocious breast development in these children, 34% of our study cohort was OW, which seems to be higher than the prevalence of OW/obesity in Korean girls [19,20], and they were significantly younger than the children in the NW group. OW girls showed more advanced BA at presentation; however, progression to central puberty occurred at similar ages between the groups, suggesting that pubertal progression might be slower in OW girls.
Many studies have reported a relationship between obesity and an earlier age of pubertal onset, and some have reported earlier age of menarche in obese girls [21,22]. However, a recent meta-analysis reported finding no difference in the age of menarche between girls who were obese and those who were normal weight [7]. In our study, OW girls were less likely than NW girls to progress to central puberty before 9 years of age, supporting that pubertal progression might be slower and the age of menarche be similar or only slightly younger in OW girls than in NW girls.
The BA advancement in OW girls could be due to increased estrogen production [23] and increased aromatization of androgens into estrogen in adipose tissue. Additionally, hyperinsulinemia and high leptin levels can contribute to bone maturation in obese children. Hyperinsulinemia stimulates growth by acting on the insulin-like growth factor-1 receptor, and leptin acts as a skeletal growth factor [1]. The OW group in this study had more advanced BA at their initial workup and at their progression to central puberty than the NW group. That could lead to compromised pubertal height gain in OW children, and further study is required to compare their final heights with those of NW children.
The peak LH level in the LHRH test tended to be lower in obese girls. It has been thought that obesity causes a relative suppression of LH levels, and because of that, obese girls with breast budding might enter central puberty later than nonoverweight girls [24]. In this study, being OW/obese was unlikely to be associated with the risk of progressing to central puberty before age 9, and progression-free survival was significantly higher in the OW group than the NW group.
However, pubertal progression was not slow in all OW girls, and it progressed rapidly in a subgroup of these children. OW girls with rapid pubertal progression had more advanced BA and higher LH, FSH, and E2 levels at their initial work-ups than OW girls with slow pubertal progression in this study. Our results suggest that although pubertal progression is slower in OW girls than NW girls, those with advanced BA and elevated hormone levels, especially an increased initial LH level, should be followed carefully, and timely intervention should be provided when required.
Within the OW group, although CA and BA at the onset of central puberty were older in the OW-SP group, BA advancement at central pubertal onset did not differ significantly between the OW-RP and OW-SP groups. BA advancement itself should be a result, rather than a cause, of pubertal progression, and when a certain number of stimuli has accumulated, the hypothalamic-pituitary-gonadal axis begins to be activated. A threshold effect for central puberty progression can be supposed, and BA advancement by 18 to 21 months might be used as a surrogate marker for the timing of central puberty progression.
When central puberty was strongly suspected by the progression of breast development and basal LH elevation, we deemed the LHRH test to be unnecessary, especially in children aged 9 years or older. Actually, even basal LH measurement was not performed in many children after 9 years of age; however, they were excluded from this study because we enrolled only laboratory-confirmed cases. To satisfy the reimbursement criteria in Korea, the LHRH test was more likely to be performed in girls younger than 9 years than in girls older than that.
Adipomastia in OW children can be misidentified as precocious breast development by parents. When precocious breast development was deemed unlikely after history taking and a physical examination, observation without laboratory workup was recommended, and parents were given brief advice on lifestyle modifications. Of course, those children were excluded from this study.
This study has some limitations. First, the sample size of OW girls with precocious puberty was relatively small, and the sample with pubertal progression was even smaller. Second, this is a single-center retrospective cohort study. Participants were enrolled from the patient database of our hospital and might not represent the general population. Third, parental anxiety and preference might have influenced the number of clinic visits and/or the aggressiveness of the workup. Fourth, the duration of follow-up might be insufficient, and we have no information about those who were lost to follow-up, which could have biased our study results.
In conclusion, pubertal progression in OW girls with precocious pubertal development seems to be slower than that in their NW counterparts. However, it can progress rapidly in OW girls with pronounced BA advancement and high gonadotropin and sex steroid levels at their initial workup.
Notes
Funding
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Fig.┬Ā1.
Flowchart of pubertal progression in overweight and nonoverweight girls with precocious breast development. LHRH, luteinizing hormone releasing hormone; BMI, body mass index; LH, luteinizing hormone.
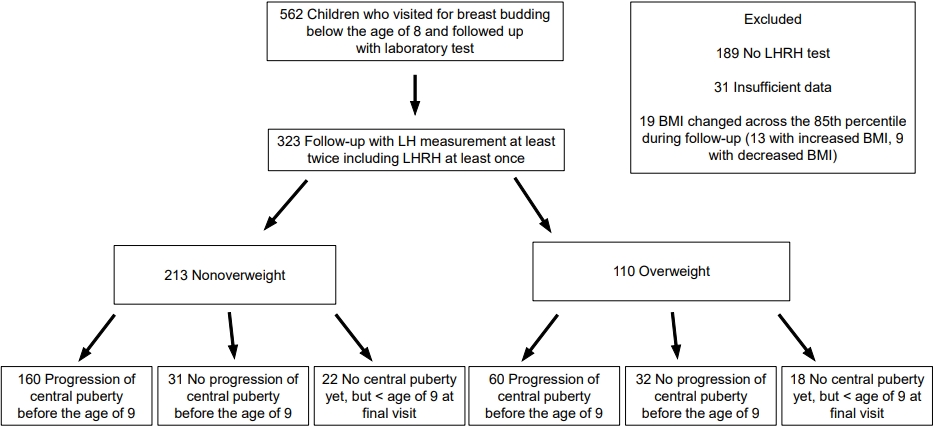
Fig.┬Ā2.
Kaplan-Meier curve showing slower progression to central puberty in overweight girls (P<0.001). OW, overweight; NW, nonoverweight; F/U, follow-up.
Table┬Ā1.
Comparison of clinical and laboratory data between the nonoverweight and overweight groups at initial workup and when progression to central puberty was first confirmed
Table┬Ā2.
Comparison of clinical and laboratory data between overweight girls with central puberty progression before 9 years of age (OW-RP group) and those without (OW-SP group)
Table┬Ā3.
Logistic regression analysis to identify predictors for the progression to central puberty before 9 years of age
References
1. Cavarzere P, Mauro M, Gaudino R, Micciolo R, Piacentini G, Antoniazzi F. Role of body weight in the onset and the progression of idiopathic premature pubarche. Horm Res Paediatr 2020;93:351ŌĆō60.



2. Wang Y. Is obesity associated with early sexual maturation? A comparison of the association in American boys versus girls. Pediatrics 2002;110:903ŌĆō10.



3. Tenedero CB, Oei K, Palmert MR. An approach to the evaluation and management of the obese child with early puberty. J Endocr Soc 2021;6:bvab173.




4. Abou El Ella SS, BarseeEm NF, Tawfik MA, Ahmed AF. BMI relationship to the onset of puberty: assessment of growth parameters and sexual maturity changes in Egyptian children and adolescents of both sexes. J Pediatr Endocrinol Metab 2020;33:121ŌĆō8.


5. Biro FM, Greenspan LC, Galvez MP, Pinney SM, Teitelbaum S, Windham GC, et al. Onset of breast development in a longitudinal cohort. Pediatrics 2013;132:1019ŌĆō27.




6. Benedet J, da Silva Lopes A, Adami F, de Fragas Hinnig P, de Vasconcelos Fde A. Association of sexual maturation with excess body weight and height in children and adolescents. BMC Pediatr 2014;14:72.




7. Li W, Liu Q, Deng X, Chen Y, Liu S, Story M. Association between obesity and puberty timing: a systematic review and meta-analysis. Int J Environ Res Public Health 2017;14:1266.



8. Kim JH, Yun S, Hwang SS, Shim JO, Chae HW, Lee YJ, et al. The 2017 Korean National Growth Charts for children and adolescents: development, improvement, and prospects. Korean J Pediatr 2018;61:135ŌĆō49.




9. Marshall WA, Tanner JM. Variation in pattern of pubertal changes in girls. Arch Dis Child 1969;44:291ŌĆō5.


10. Kang YS, Yoo DY, Chung IH, Yoo EG. Diurnal variation of gonadotropin levels in girls with early stages of puberty. Ann Pediatr Endocrinol Metab 2017;22:183ŌĆō8.




11. McCartney CR, Prendergast KA, Chhabra S, Eagleson CA, Yoo R, Chang RJ, et al. The association of obesity and hyperandrogenemia during the pubertal transition in girls: obesity as a potential factor in the genesis of postpubertal hyperandrogenism. J Clin Endocrinol Metab 2006;91:1714ŌĆō22.



12. Lee DS, Ryoo NY, Lee SH, Kim S, Kim JH. Basal luteinizing hormone and follicular stimulating hormone: is it sufficient for the diagnosis of precocious puberty in girls? Ann Pediatr Endocrinol Metab 2013;18:196ŌĆō201.



13. Bizzarri C, Spadoni GL, Bottaro G, Montanari G, Giannone G, Cappa M, et al. The response to gonadotropin releasing hormone (GnRH) stimulation test does not predict the progression to true precocious puberty in girls with onset of premature thelarche in the first three years of life. J Clin Endocrinol Metab 2014;99:433ŌĆō9.


14. Brito VN, Latronico AC, Arnhold IJ, Mendon├¦a BB. Update on the etiology, diagnosis and therapeutic management of sexual precocity. Arq Bras Endocrinol Metabol 2008;52:18ŌĆō31.


15. Brito VN, Batista MC, Borges MF, Latronico AC, Kohek MB, Thirone AC, et al. Diagnostic value of fluorometric assays in the evaluation of precocious puberty. J Clin Endocrinol Metab 1999;84:3539ŌĆō44.


16. Resende EA, Lara BH, Reis JD, Ferreira BP, Pereira GA, Borges MF. Assessment of basal and gonadotropinreleasing hormone-stimulated gonadotropins by immunochemiluminometric and immunofluorometric assays in normal children. J Clin Endocrinol Metab 2007;92:1424ŌĆō9.


17. Harrington J, Palmert MR, Hamilton J. Use of local data to enhance uptake of published recommendations: an example from the diagnostic evaluation of precocious puberty. Arch Dis Child 2014;99:15ŌĆō20.


18. Tomova A. Body weight and puberty. New York: Springer; 2016:95-108.
19. Kim M, Kim J. Cardiometabolic risk factors and metabolic syndrome based on severity of obesity in Korean children and adolescents: data from the Korea National Health and Nutrition Examination Survey 2007ŌĆō2018. Ann Pediatr Endocrinol Metab 2022;27:134ŌĆō41.




20. Kang S, Seo MY, Kim SH, Park MJ. Changes in lifestyle and obesity during the COVID-19 pandemic in Korean adolescents: based on the Korea Youth Risk Behavior Survey 2019 and 2020. Ann Pediatr Endocrinol Metab 2022;27:281ŌĆō8.




21. Barros BS, Kuschnir MCMC, Bloch KV, Silva TLND. ERICA: age at menarche and its association with nutritional status. J Pediatr (Rio J) 2019;95:106ŌĆō11.


22. Shrestha A, Olsen J, Ramlau-Hansen CH, Bech BH, Nohr EA. Obesity and age at menarche. Fertil Steril 2011;95:2732ŌĆō4.


- Related articles in APEM
-
Measurement of Bone Mineral Density in Children with Normal Growth and Development.1998 May;3(1)







