The effect of hypothalamic involvement and growth hormone treatment on cardiovascular risk factors during the transition period in patients with childhood-onset craniopharyngioma
Article information
Abstract
Purpose
Hypothalamic damage may increase the risk of adulthood obesity and cardiovascular disease in patients with craniopharyngioma. We evaluated the effects of hypothalamic involvement (HI) and growth hormone (GH) discontinuation on cardiovascular risk factors during the transition period in patients with childhood-onset craniopharyngioma.
Methods
Thirty-three patients (17 males, 16 females) underwent retesting for adult GH deficiency (GHD) between 2005 and 2020 at Seoul National University Children's Hospital. Postoperative HI was graded by Puget's criteria and data regarding GH replacement were collected. At retesting, body mass index (BMI), fasting blood glucose, insulin, high-density lipoprotein cholesterol (HDL-C), triglycerides, and blood pressure were assessed.
Results
The mean age of commencement and discontinuation of GH replacement for childhood GHD was 10.0±3.6 and 15.3±3.1 years, respectively. The mean age at retesting for adult GHD was 17.7±2.5 years. When patients were categorized by post-GH discontinuation duration, those with durations >6 months (n=27) showed lower HDL-C levels than those with <6 months (P=0.037). Patients with extensive HI (n=16) had higher BMI z-scores than did those with no HI or mild HI (P=0.020). Both the extent of HI and longer post-GH discontinuation duration were significantly predictive for decreased HDL-C levels (P<0.05, for both).
Conclusions
The extent of HI and GH discontinuation duration during the transition period can increase cardiovascular risks in patients with childhood-onset craniopharyngioma.
Highlights
· In childhood-onset craniopharyngioma patients, the more extensive the hypothalamic involvement or the longer the period of growth hormone discontinuation, the higher the risk of dyslipidemia during transition period.
Introduction
Craniopharyngiomas, benign tumors in the sellar or suprasellar area of the brain, account for 3.5% to 10.5% of childhood intracranial tumors [1,2]. Although histologically benign, craniopharyngiomas are clinically malignant. The proximity to the optic nerve/chiasma and hypothalamic-pituitary axis can cause sequelae, and the recurrence rate in infants and children is high [3]. The tumor itself or treatment-associated hypothalamic damage and hypopituitarism can contribute to long-term cardiovascular complications [4,5]. In particular, patients with craniopharyngiomas show higher rates of metabolic syndrome than do those with pituitary adenomas or other tumors that invade the pituitary gland [6].
Studies have emphasized the close association between preoperative invasion and/or treatment-associated hypothalamic injury and obesity [7-9]. One report also suggested a relationship between hypothalamic involvement (HI) and increased cardiovascular risk factors in adult patients with childhood-onset craniopharyngioma [10]. Damage to the hypothalamus and pituitary gland can result in multiple pituitary hormone deficiencies (MPHDs), including those involving growth hormone (GH), adrenocorticotropic hormone (ACTH), thyroid-stimulating hormone (TSH) and/or gonadotropins. Adverse effects of GH deficiency (GHD) or MPHD on lipid profiles, glucose homeostasis, and even cardiac dimensions and function have been reported [11-13]. Continued GH replacement could benefit body composition, as shown by increases in lean body mass and decreases in fat mass in patients with GHD receiving GH replacement [14,15]. Investigations into whether cessation of GH at adult height results in adverse cardiometabolic effects in patients with childhood-onset GHD found that the cessation and the duration of discontinuation may affect cardiovascular risk factors [16,17]. In contrast, a previous study in Korea reported no significant correlations between GH interruption during the transition period and the lipid profiles. However, the study did not measure other parameters related to cardiovascular risks, such as blood pressure and glucose [18].
In this study, we assessed both the extent of HI and the duration of GH discontinuation as factors associated with long-term cardiovascular risks in patients with childhood-onset craniopharyngioma. We hypothesized that both factors may be associated with changes in metabolic syndrome components. We measured obesity, fasting glucose, lipid profiles, and blood pressure after completion of linear growth during the transition period and evaluated their association with the extensiveness of postoperative HI and the duration of time after GH discontinuation.
Materials and methods
1. Subjects
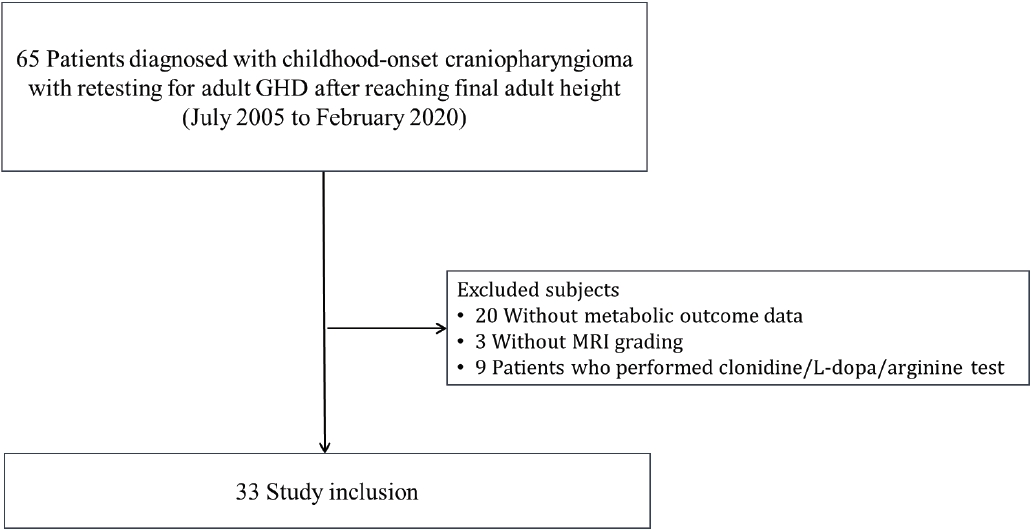
Medical records of previous diagnostic tests and treatment courses, including operations, radiation therapy, hormone testing, hormone replacement medications, brain imaging, cardiovascular risk factors, and a family history of cardiovascular disease, were reviewed retrospectively. Among patients diagnosed with childhood-onset craniopharyngioma at Seoul National University Children’s Hospital from 1995 to 2012, 65 patients who received retesting for adult GHD after reaching their final adult height between 2005 and 2020 were included (Fig. 1). Twenty-three patients were excluded due to missing data for either assessments of cardiovascular risk factors (n=20) or postoperative HI grade (n=3). Nine patients who underwent GH stimulation tests using either clonidine, L-dopa, or arginine were also excluded. Finally, 33 patients were included in the study analyses.
2. Postoperative classification of HI
Postoperative magnetic resonance imaging was used by 2 pediatric radiologists to assess the extent of postoperative HI as described previously [9] and according to Puget's grading system [19]. Patients were classified as having "none" (no), "mild" (negligible or residual tumor displacing the hypothalamus) or "extensive" (unidentifiable floor of the third ventricle) postoperative HI. For this study, patients with no or mild involvement were classified into the same none/mild group due to the small number of subjects. Analyses of HI compared the none/mild and extensive groups in this study.
3. GH retesting to diagnose adulthood GHD during the transition period
Retesting to confirm adult GHD was conducted during the transition period in patients who had previously received GH for GHD following childhood-onset craniopharyngioma. An insulin tolerance test was used as the standard test for GH retesting. Patients with stimulated GH peaks of less than 5 μg/L were defined as having adult GHD. For analyses regarding the duration of GH discontinuation, the time between the last GH treatment to GH retesting was calculated. According to the calculated duration of GH discontinuation, patients were classified as having discontinued GH for less than or more than 6 months.
4. Evaluation of anthropometrics and metabolic syndrome components.
On the day of GH retesting, height was measured using a Harpenden stadiometer (Holtain Ltd., Crosswell, UK) and weight was measured using a digital scale. Height, weight, and body mass index (BMI) were used to calculate age- and sex-specific z-scores according to the 2017 Korean national growth charts [20]. Obesity was defined by a BMI ≥ 95th percentile for age and sex in children and adolescents, and by an absolute value of BMI ≥25 kg/m2 in adults [21]. Central obesity was defined as a waist circumference (WC) ≥90th percentile for age- and sex-specific references in children [22] and as an absolute WC ≥90 cm in adult males or ≥80 cm in adult females [21]. Blood pressure was measured using an automated device.
Blood samples were obtained from subjects after they had fasted for 12 hours. The samples were measured for levels of serum glucose, insulin, total cholesterol, triglycerides, and high-density lipoprotein cholesterol (HDL-C). Insulin resistance (IR) was assessed by the homeostatic model assessment for IR using the following formula: fasting insulin (μIU/mL)×fasting blood sugar (mg/dL)/405. The presence of metabolic syndrome was determined according to the International Diabetes Federation criteria [23] with central obesity plus 2 or more of the following: impaired fasting glucose (≥100 mg/dL), high triglycerides (≥150 mg/dL), low HDL-C (<40 mg/dL for males and <50 mg/dL for females), and/or hypertension (systolic blood pressure ≥130 mmHg or diastolic blood pressure ≥85 mmHg).
5. Statistical analyses
All statistical analyses were performed using IBM SPSS Statistics version 25.0 (IBM Co., Armonk, NY, USA). All continuous variables were tested for normality and described as mean±standard deviation for normally distributed data, or median and range for nonparametric data. For analyses of cardiovascular risk factors with nonnormal distribution, triglycerides, fasting glucose, fasting insulin, and homeostatic model assessment of IR were logarithm-transformed to approximate a normal distribution. Differences and associations between cardiovascular risk factors as dependent variables and HI (none/mild vs. extensive) and post-GH duration (<6 months vs. ≥6 months) groups as independent factors of interest were analyzed. Student t-tests or Mann-Whitney U-tests were used to compare continuous variables, while chi-squared tests or Fisher exact tests were used to compare categorical variables between 2 independent groups. The magnitude of the difference in mean values between groups was determined by effect size, using partial eta squared (denoted as η2). Two-way analysis of variance (ANOVA) was conducted to analyze the effects of the main factors (HI and post-GH duration) and their interaction with each of the cardiovascular risk factors. Covariates were considered in models using analysis of covariance (ANCOVA), which included each of the cardiovascular risk factors as a dependent variable, HI and post-GH duration as the main factors, and the following covariates based on previous literature [5,8,17]: age at the start of GH therapy, duration of childhood GH therapy, age at retesting, sex (male=0, female=1), postoperative duration, and family history of cardiometabolic disease (no=0, yes=1). Because the interaction term (HI×post-GH duration) was significant for HDL-C in an analysis by 2-way ANOVA, it was included in the ANCOVA models. Statistical significance was defined as P<0.05.
6. Ethical statement
The Institutional Review Board of Seoul National University Hospital (approval number: 2007-193-1144) approved the study and written informed consent was waived due to the retrospective nature of the study.
Results
1. Clinical characteristics
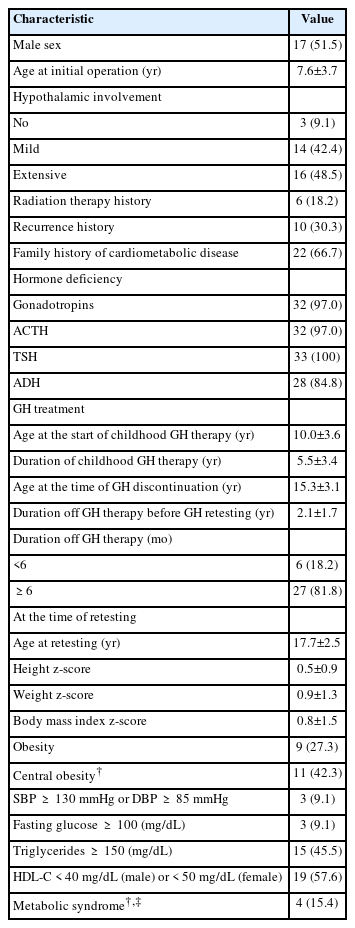
The clinical characteristics of the 33 patients (17 males and 16 females) are described in Table 1. The mean age of the patients was 7.6±3.7 years at the initial operation. The degree of postoperative HI was none/mild in 17 patients (51.5%) and extensive in 16 patients (48.5%). Six patients received radiation therapy. Coexisting hormone deficiencies were as follows: GH (n=33, 100%), gonadotropins (n=32, 97%), TSH (n=33, 100%), ACTH (n= 32, 97%), and antidiuretic hormone (n=28, 84.8%).
All patients were treated with GH for childhood GHD and had discontinued treatment by the time of retesting. The respective ages at the commencement and discontinuation of GH replacement were 10.0±3.6 years and 15.3±3.1 years. Among the subjects, 14 patients stopped GH therapy after reaching their final adult height. However, GH therapy was discontinued before completion of linear growth in 19 patients; 11 patients discontinued after reaching 150 cm (females) or 160 cm (males), cutoff heights after which the cost of GH therapy is no longer covered by national health insurance. The other 8 patients discontinued GH therapy before completion of linear growth, due to recurrences (n=3), nonadherence (n=3), or adverse effects (n=2). The mean post-GH duration was 2.1±1.7 years for the total group, with 27 patients (81.8%) discontinuing GH treatments for more than 6 months (mean 2.6±1.6 years) and 6 patients (18.2%) doing so for less than 6 months (mean 0.2±0.2 years) (Table 2).
2. Measures of anthropometrics and metabolic syndrome components
The mean age at GH retesting was 17.7±2.5 years, and all patients were diagnosed with adult GHD. The mean final height z-score at the time of retesting was 0.5±0.9 and the mean BMI z-score was 0.9±1.3. Among all subjects, 9 (27.3%) were obese. Of the 26 patients with available measures of WC, 11 (42.3%) were centrally obese. Metabolic syndrome was present in 4 patients (15.4%). The numbers of patients with impaired fasting glucose, high triglycerides, low HDL-C, and hypertension were 3 (9.1%), 15 (45.5%), 19 (57.6%), and 3 (9.1%), respectively (Table 1).
3. Association of postoperative HI and post-GH discontinuation duration with cardiovascular risk factors
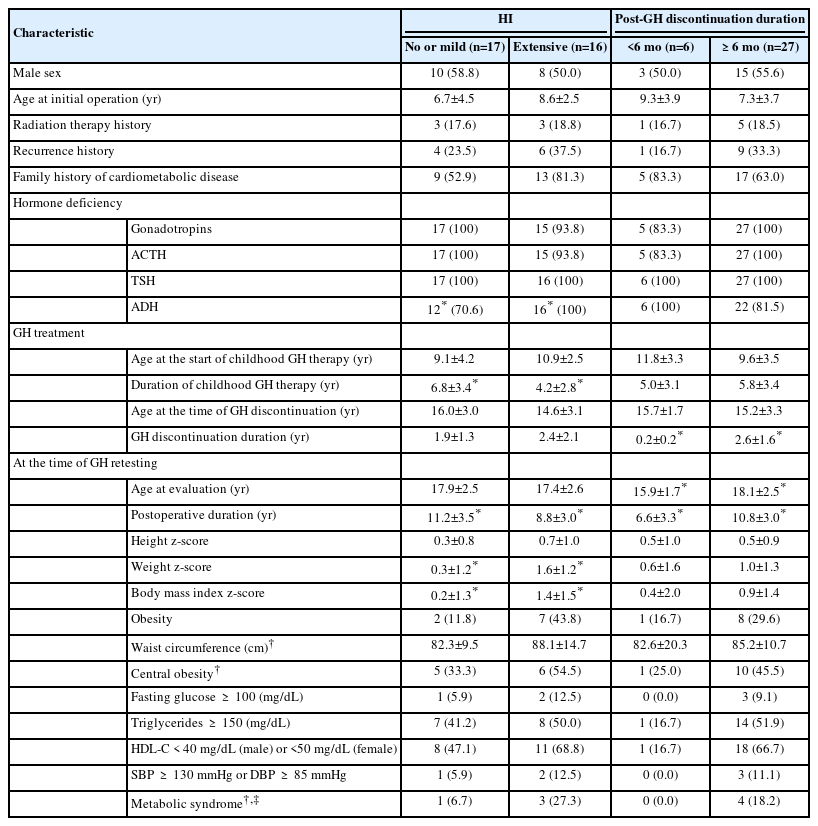
First, cardiovascular risk factors were compared according to postoperative HI status. BMI z-scores (mean 1.4 vs. 0.2, P=0.020) and weight z-scores (mean 1.6 vs. 0.3, P=0.007) were both significantly higher in the extensive HI group compared with the none/mild HI group (Tables 2, 3). When cardiovascular risk factors were compared according to the duration of GH discontinuation, patients with post-GH durations of more than 6 months (n=27) showed significantly lower HDL-C levels (mean 39.9 mg/dL vs. 51.8 mg/dL, P=0.037) compared with those within 6 months of GH discontinuation. BMI z-scores and other components of metabolic syndrome did not differ significantly between the 2 groups (Table 3).
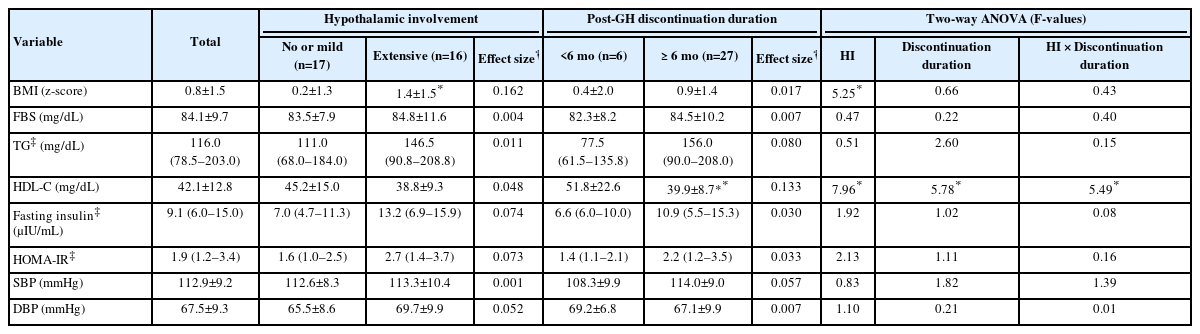
Cardiovascular risk factors according to the extent of hypothalamic involvement and GH discontinuation duration
Two-way ANOVA was conducted to examine the relationship of the 2 main factors (HI and GH discontinuation duration) and their interaction effect with cardiovascular risk factors (Table 3). The interaction effect (HI×GH discontinuation duration) was significant for HDL-C (F=5.488, P=0.026). The main effect of HI on BMI z-score and HDL-C was statistically significant (F=5.254, P=0.029 for BMI z-score; F=7.959, P=0.009 for HDL-C). The main effect of GH discontinuation duration on HDL-C was also statistically significant (F=5.781, P=0.023 for HDL-C) (Table 3).
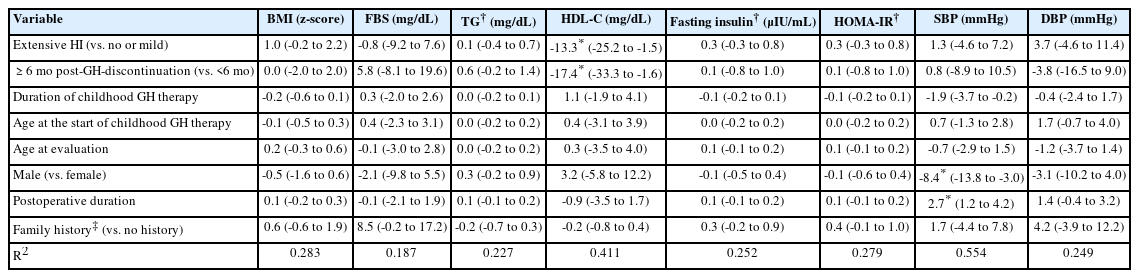
ANCOVA was constructed to investigate the effect of the duration of HI and GH discontinuation on each of the cardiovascular risk factors after adjusting for age at the start of GH therapy, duration of childhood GH therapy, sex, age at retesting, postoperative duration, and a family history of cardiovascular disease. More extensive HI was significantly predictive of decreased HDL-C levels (mean difference [MD]=-13.3, P=0.029). Longer post-GH discontinuation duration was also significantly predictive of decreased HDL-C levels (MD=-17.4, P=0.032) (Table 4).
Discussion
During the transition period, impaired fasting glucose or hypertension were detected in one-tenth, and dyslipidemia in more than half, of the patients with childhood-onset craniopharyngioma. Extensive HI and a longer period of GH discontinuation were associated with lower HDL-C at the time of adult GHD testing in patients treated for childhood-onset craniopharyngioma.
The prevalence of metabolic syndrome at the time of GH retesting during the transition period was 15.4% in our patients with craniopharyngioma. The prevalence of metabolic syndrome in adult patients with childhood-onset craniopharyngioma has been reported to be within a range of 25% to 46% in previous studies [5,10,24], which is higher than that of our study. Possible reasons for the variations in reported prevalence may be due to differences in age at the time of evaluation, proportion of obese patients, and regional differences. When compared with population-based reports with a prevalence of metabolic syndrome of 1.7% to 2.2% of adolescents [25], metabolic syndrome prevalence was much higher in our study patients. Concerning lipid metabolism, the proportions of patients with low HDL-C (58%) and hypertriglyceridemia (46%) according to the International Diabetes Federation [23] criteria were both higher than those reported in populations of healthy Korean adolescents [25]. Pediatric patients with craniopharyngioma (7–18 years old) have been reported to exhibit worse lipid profiles compared with control subjects matched for age, sex, BMI, and pubertal stage [26,27], which is in line with our study results. The higher prevalence of metabolic syndrome and dyslipidemia in patients treated for childhood-onset craniopharyngioma may contribute to higher long-term morbidity and mortality. As such, as the patient progresses to adulthood, efforts to prevent, monitor, and treat metabolic syndrome and its components are necessary.
When we evaluated each component of metabolic syndrome during the transition period, patients with extensive HI showed higher BMI z-scores, but the difference was not significant when adjusted for the duration of post-GH discontinuation. Extensive HI was significantly predictive of decreased HDL-C with adjustment for covariates. A study of adolescents and young adults with childhood-onset craniopharyngioma on complete GH replacement by Holmer et al. [10] reported a higher cardiovascular risk in those with tumor growth into the third ventricle. Our study corroborates such findings, with positive correlations observed between the BMI z-score and HI. The tumor itself or treatment-associated damage in several hypothalamic nuclei such as the periventricular nucleus, the arcuate nucleus, and ventromedial nucleus could result in leptin resistance, IR, and autonomic disturbance, leading to obesity and increased cardiometabolic risk [5,28]. Considering the morbidities associated with childhood-onset craniopharyngioma, several studies have suggested using grading systems to assess hypothalamic damage and individualized and multidisciplinary approaches to treatment [19,29,30]. Taken together, these results emphasize that avoiding irreversible hypothalamic damage may be key to reducing long-term cardiovascular risks in childhood-onset craniopharyngioma patients.
When evaluating the effect of GH cessation on cardiometabolic risk, a longer duration of post-GH discontinuation was significantly predictive for decreased HDL-C. Replacement of GH in adults and children with GHD has been shown to reduce cardiovascular risks by preventing deterioration of the lipid profile [11,31] or by improving cardiac dimensions and function [13]. GH induction of lipolysis is a possible mechanism through which GH can affect lipid metabolism [32]. GH stimulates lipolysis in fat depots, and enriched production of apoE and apoB48 by GH results in rapid clearance of very low-density lipoprotein (LDL), which may affect the synthesis of LDL [33]. Moreover, GH directly affects expression of the hepatic LDL receptor, resulting in accelerated clearance of LDL cholesterol (LDL-C) by GH treatment [34,35]. The beneficial effects of GH replacement on lipid profiles in GH-deficient adults have been reported in a meta-analysis [31]; however, only total cholesterol and LDL-C levels were significantly decreased. A study of 186 patients with nonidiopathic GHD showed that a longer duration of GH cessation during the transition phase was associated with a worse lipid profile; in particular, higher levels of total cholesterol, triglycerides, and LDL-C [17]. Furthermore, prospective studies have shown that replacement of GH was significant in increasing HDL-C levels in adult GHD patients [14,36]. A study of 45 patients with childhood-onset GHD due to various causes [18] who received GH replacement for adult GHD also showed increases in HDL-C and decreases in LDL-C after 1 year. However, the same study reported no significant correlations between the duration of GH treatment interruption with baseline lipid profile during the transition period, which contrasts with our results of decreased HDL-C in those with more than 6 months of GH discontinuation. The consequences of an isolated low HDL-C measurement on cardiovascular risk has been reported in a long-term follow-up study of patients that had low HDL-C levels with normal LDL-C and total cholesterol. It was observed that men with isolated low HDL-C levels had a higher risk of coronary heart disease compared with men with normal HDL-C levels, with an estimated risk ratio of 1.24 [37]. Consequently, interruptions in GH treatment should cause concern about long-term cardiometabolic risk, particularly in adults with severe hypothalamic damage.
More than half of the patients in our study discontinued GH therapy due to the limited insurance coverage of GH implemented by our country's Health Insurance Review Assessment Service. Other possible reasons for discontinuation include concerns about cost-effectiveness, nonadherence, and adverse effects [38]. However, even after reaching final adult height, continuous GH treatments may improve hormonal health and quality of life in adulthood. Our study contributes evidence regarding the adverse effects of GH cessation during the transition period on the lipid profile in patients with childhood-onset craniopharyngioma. Recently, the Korean Endocrine Society and the Korean Society of Pediatric Endocrinology released a position statement on GHD [39], emphasizing the importance of continued GH replacement during the transition period, and pressing for continuous insurance coverage of GH until the completion of linear growth. Taken together, GH interruption should be avoided during the transition period before completion of linear growth and early recommencement of GH should be considered immediately after the confirmation of adult GHD.
Our study has several limitations. The power and generalizability of this study were limited by its small sample size and retrospective design, which also made it difficult to assess the associations between cardiometabolic risk and the duration of GH discontinuation as a continuous variable. Furthermore, we could not compare cardiovascular risk factors between patients and healthy controls due to the lack of a control group. We also could not compare differences in the proportions of metabolic syndrome according to HI or periods of GH cessation due to missing data on WC. Nevertheless, this study is the first to analyze both the degree of HI and the duration of post-GH discontinuation, with parameters of cardiometabolic risk in patients with childhood-onset craniopharyngioma during the transition to adulthood.
In conclusion, dyslipidemia was present at the time of GH retesting during the transition period in approximately half of patients with childhood-onset GHD due to craniopharyngiomas. Considering the higher risk of dyslipidemia in cases with longer periods of GH cessation and/or extensive HI, continuation of GH treatment in adults with childhood organic GHD patients and treatment strategies to minimize hypothalamic injury are strongly required.
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Funding
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data availability
The data that support the findings of this study can be provided by the corresponding author upon reasonable request.
Author contribution
Conceptualization: CHS, HWJ, YAL; Data curation: SHP, JEC, YAL; Formal analysis: SHP, HWJ, YAL; Methodology: CHS, HWJ, YAL; Project administration: CHS, YAL; Visualization: SHP, HWJ; Writing - original draft: SHP, HWJ, YAL; Writing - review & editing: YJL, HWJ, YAL