 |
 |
- Search
| Ann Pediatr Endocrinol Metab > Volume 27(3); 2022 > Article |
|
Abstract
Purpose
Methods
Results
Conclusions
Notes
Funding
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Fig. 1.
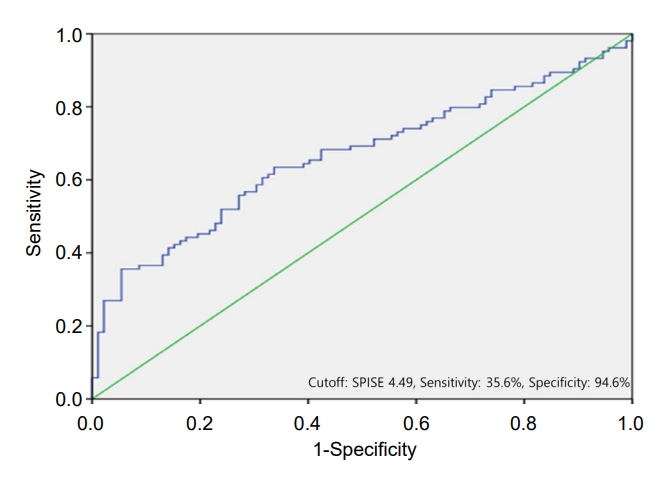
Table 1.
| Characteristic | NO (n=92) | Prediabetes (n=59) | T2DM (n=104) | P-value |
|---|---|---|---|---|
| Age at diagnosis (yr) | 14.01±1.93 | 13.25±2.17 | 14.42±1.91† | <0.01a) |
| Male sex | 54 (58.6) | 31 (52.5) | 60 (57.7) | 0.742b) |
| BMI at diagnosis (kg/m2) | 27.20±2.98 | 27.88±2.86 | 28.39±4.62 | 0.082a) |
| BMI z-score at diagnosis | 2.34± 0.97 | 2.73± 1.00 | 2.73±1.77 | 0.093a) |
| Family history of T2DM | 42 (45.7) | 28 (47.4) | 77 (74.0)*,† | <0.01b) |
| Fatty liver | 26 (28.3) | 28 (47.4)* | 76 (73.1)*,† | <0.01b) |
| Acanthosis nigricans | 61 (66.3) | 43 (72.9) | 73 (70.2) | 0.679b) |
Table 2.
| Characteristic | NO (n=92) | Prediabetes (n=59) | T2DM (n=104) | P-value |
|---|---|---|---|---|
| Glucose at present (mg/dL) | 94.36±8.76 | 95.56±8.57 | 179.84±69.82* | <0.01 |
| HbA1c at present (%) | 5.27±0.26 | 5.78±0.22* | 9.98±2.58*,† | <0.01 |
| AST (mg/dL) | 23.37±9.44 | 32.49±22.20* | 46.23±36.38*,† | <0.01 |
| ALT (mg/dL) | 26.21±17.98 | 47.71±47.60* | 70.25±62.05*,† | <0.01 |
| Total cholesterol (mg/dL) | 168.07±30.00 | 176.76±29.92 | 195.35±42.57*,† | <0.01 |
| Triglyceride (mg/dL) | 111.40±55.24 | 124.54±68.84 | 164.99±108.17*,† | <0.01 |
| HDL-Cholesterol (mg/dL) | 47.82±11.49 | 45.84±9.07 | 42.15±8.65*,† | <0.01 |
| LDL-Cholesterol (mg/dL) | 101.24±23.51 | 108.04±27.14 | 125.29± 37.80*,† | <0.01 |
| HOMA-IR | 4.95±2.92 | 6.26±3.99 | 8.42±6.34*,† | <0.01 |
| SPISE | 6.01±1.19 | 5.67±1.13 | 5.33±1.54* | <0.01 |
Values are presented as mean±standard deviation.
NO, normal overweight/obesity; T2DM, type 2 diabetes mellitus; HbA1c, glycosylated hemoglobin; HOMA-IR, homeostatic model assessment of insulin resistance; AST, aspartate aminotransferase; ALT, alanine aminotransferase; SPISE-IR, single point insulin sensitivity estimator of insulin resistance.
P-values were calculated by analysis of variance. For multiple comparisons, Bonferroni post hoc test was performed.
Table 3.
| Variable |
Unadjusted model |
Adjusted model |
||
|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | |
| Age | 1.009 (0.997–1.022) | 0.138 | 1.007 (0.994–1.025) | 0.425 |
| Male sex | 1.042 (0.590–1.841) | 0.887 | 0.806 (0.339–1.918) | 0.626 |
| BMI SDS | 1.210 (0.987–1.484) | 0.066 | 0.458 (0.304–0.691) | <0.01 |
| Family history of T2DM | 3.395 (1.863–6.189) | <0.01 | 3.812 (1.585–9.166) | <0.01 |
| Fatty liver | 6.890 (3.679–12.904) | <0.01 | 4.631 (1.848–11.603) | <0.01 |
| AST | 1.058 (1.033–1.083) | <0.01 | 0.998 (0.946–1.053) | 0.949 |
| ALT | 1.305 (1.021–1.049) | <0.01 | 1.029 (0.998–1.062) | 0.069 |
| Triglyceride | 1.010 (1.005–1.016) | <0.01 | 1.002 (0.996– 1.008) | 0.547 |
| HDL-cholesterol | 0.943 (0.914–0.974) | <0.01 | 0.987 (0.945–1.030) | 0.552 |
| HOMA-IR >cutoff point† | 2.160 (1.218–3.832) | <0.01 | 1.217 (0.512–2.891) | 0.657 |
| SPISE<4.49 | 9.609 (3.582–25.775) | < 0.01 | 50.307 (8.053–314.255) | <0.01 |
AST, aspartate aminotransferase; ALT, alanine aminotransferase; TG, triglyceride; HDL, high-density lipoprotein; CI, confidence interval; OR, odds ratio; BMI, body mass index SDS, standard deviation score; T2DM, type 2 diabetes mellitus; HOMA-IR, homeostatic model assessment of insulin resistance; SPISE, single point insulin sensitivity estimator.
References
- TOOLS







