Effect of body mass index on peak growth hormone level after growth hormone stimulation test in children with short stature
Article information
Abstract
Purpose
The aim of this study is to evaluate the effect of body mass index (BMI) on peak serum growth hormone (GH) level after GH stimulation test in children with short stature.
Methods
Data were obtained from retrospective medical record reviews of those who visited the pediatric endocrine clinic at St. Vincent’s Hospital of Catholic University for short stature from January 2010 to June 2019. A total of 115 children (66 boys and 49 girls) whose height was less than the third percentile according to age and sex underwent GH stimulation testing.
Results
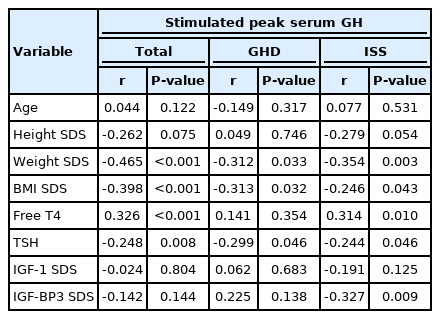
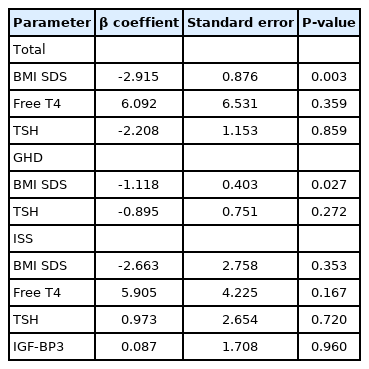
Of the 115 subjects, 47 were diagnosed with GH deficiency (GHD) and 68 were diagnosed with idiopathic short stature (ISS). In patients with GHD, weight standard deviation score (SDS) (P<0.001) and BMI SDS (P≤0.001) were higher, and free thyroxine (T4) level (P=0.012) was lower than those in the ISS group. In total subjects, peak serum GH level after GH stimulation test showed negative correlations with weight SDS (r=-0.465, P<0.001), BMI SDS (r=-0.398, P<0.001), and thyroid stimulating hormone (r=-0.248, P=0.008) and a positive correlation with free T4 (r=0.326, P<0.001). In multiple regression analysis, BMI SDS (P=0.003) was negatively associated with peak serum GH level in GH stimulation testing after adjusting for age, sex, pubertal status, and type of pharmacological stimulus.
Conclusions
The BMI SDS influences peak serum GH level after GH stimulation testing. We should consider BMI factors when interpreting the results of GH stimulation testing.
Highlights
This study evaluated the effect of BMI on peak serum GH level after GH stimulation testing in short stature. The results showed that BMI SDS was negatively associated with peak serum GH. Consideration for BMI factor is needed when interpreting the results of GH stimulation testing in short stature.
Introduction
Short stature is defined as a height less than -2 standard deviations (SDs) or less than 3 percentile ranks according to age and sex. These children are subjects for growth hormone (GH) stimulation testing to distinguish between GH deficiency (GHD) and idiopathic short stature (ISS). The diagnosis of GHD is established when peak serum GH level does not reach an arbitrary cutoff value (peak GH<10 ng/mL) in 2 GH stimulation tests using different stimuli [1,2].
Hormonal and physiologic factors are important for GH secretions. Hormonal control typically is regulated by 2 counteractive hormones, GH-releasing hormone (GHRH) and somatostatin, known to stimulate and inhibit the release of GH from the pituitary gland, respectively. The feedback system also affects GH secretion, either by GH itself or by insulin-like growth factor 1 (IGF-1). Hormonal regulation of GH secretion is determined by these 2 processes.
Physiologic factors involved in the secretion of GH include age, onset of puberty, body weight, and so on [3]. It is known that GH secretion is particularly sensitive to nutritional factors and obesity. Several previous studies have reported that obesity can decrease spontaneous [4,5] and stimulated [6,7] GH secretion in adults. Likewise, obese children also exhibit decreased spontaneous [8,9] and diminished peak GH in GH stimulation testing. A negative correlation between body mass index (BMI) and peak GH response in stimulation testing has been reported [10,11].
However, previous studies were focused on a specific group or stimulant, making it difficult to understand the effect of BMI on peak serum GH level according to group classified by diagnosis or stimulants. Therefore, the aim of this study was to evaluate the effect of BMI on peak serum GH level after GH stimulation testing in children with short stature divided into GHD and ISS groups and the type of stimulants. We interpret the results of GH stimulation testing considering the patient’s physiologic status through this study.
Materials and methods
1. Subjects
Data were obtained from retrospective medical record reviews of those who visited the pediatric endocrine clinic at St. Vincent Hospital of Catholic University for short stature from January 2010 to June 2019. We studied 115 children (aged 3–17 years, 66 boys and 49 girls) whose height was less than the third percentile for age and sex. Patients who had other autoimmune, hematologic, or endocrine diseases were excluded. Birth weight, parental height, chronologic age, bone age, height standard deviation score (SDS), weight SDS, BMI SDS, free thyroxine (T4), triiodothyronine), thyroid stimulating hormone (TSH), cortisol, adrenocorticotropic hormone, GH, IGF-1 SDS, insulinlike growth factor binding protein-3 (IGF-BP3) SDS, and peak serum stimulated GH were analyzed.
2. Stuy design
GH stimulation tests were performed in the morning after overnight fasting. The subjects of this study underwent GH stimulation testing with combinations of 2 of the following 5 stimulants to assess GH secretion: levodopa (Myung In Pharm, Seoul, Korea; <15 kg: 150 mg; 15–35 kg: 250 mg; >35 kg: 500 mg), clonidine (HK innoN, Seoul, Korea; 150 μg/m2), insulin (Lilly, Seoul, Korea; 0.05 unit/kg), glucagon (Boryung Pharm, Seoul, Korea; 30 μg/kg, max 1 mg), and arginine (Green Cross Well Being, Bundang, Korea; 0.5 g/kg, max 30 g). Two stimulants were tested at intervals of more than 24 hours. Blood samples were collected at the time of administration of the stimuli and at 30, 60, 90, and 120 minutes after administration of stimuli to measure peak serum GH level. GHD was defined when peak serum GH level was less than 10 ng/mL after stimulation with a combination of 2 separate simulants [1]. If peak serum GH level exceeded 10 ng/mL in even one stimulation test, the child was diagnosed with ISS.
3. Anthropometric and laboratory measurements
Height was measured to 0.1 cm with a Harpenden Stadiometer (Holtain Ltd., Wales, UK) and weight was measured to 0.1 kg using a CAS scale (CAS, Seoul, Korea). All anthropometry data were calculated to a z-score based on 2017 Korean National Growth chart reference data [12]. Serum GH level was measured with immunoradiometric assay (Immulite 2000, Siemens Medical Solutions Diagnostics, Bracknell, UK). Obese in this study means BMI > 85th percentile for one's age and sex, while puberty was defined as more advanced than sex maturity rating 2.
4. Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics ver. 24.0 (IBM Co., Armonk, NY, USA). Comparisons between groups were performed using independent t-test or Mann-Whitney U-test and Pearson chi-square test. Correlation analysis was performed using the Pearson correlation coefficient. Univariate linear regression analysis was performed to determine variables associated with peak GH. Multiple regression analysis was used to identify independent predictors of peak GH response to stimulants. Significance was defined as P<0.05. Results were described as mean±standard deviation unless otherwise stated.
Results
1. Clinical and biochemical characteristics of the subjects
The study population included 66 males (57.4%) and 49 females (42.6%) with a mean age of 9.06±3.35 years. Of the 115 patients, 47 (40.9%) were diagnosed with GHD and 68 (59.1%) were diagnosed with ISS. Patients diagnosed with GHD had higher weight SDS (P<0.001) and BMI SDS (P≤0.001) and lower free T4 level than patients diagnosed with ISS (P=0.012). There was no statistically significant difference in TSH, GH, IGF-1 SDS, and IGF-BP3 between the GHD and ISS groups (Table 1).
2. Comparison of peak serum GH level after GH stimulation test by type of stimulant
For the 115 subjects, a total of 225 GH stimulation tests was performed (in 5 cases, we confirmed that the peak GH level caused by the first stimulant was greater than 10 ng/mL in that day, so a second study was not performed). Dopamine, clonidine, glucagon, insulin, and arginine were used in 111 (49%), 7 (3%), 19 (9%), 56 (25%), and 32 cases (14%), respectively. Peak serum GH level in the GHD group simulated by dopamine (P<0.001), glucagon (P=0.011), insulin (P<0.001), and arginine (P<0.001) was lower than that in the ISS group (Table 2).
3. Correlations between variables and peak serum GH level after GH stimulation testing
Peak serum GH level after GH stimulation testing exhibited negative correlations with weight SDS (r=-0.465, P<0.001), BMI SDS (r=-0.398, P<0.001), and TSH (r=-0.248, P=0.008). On the other hand, free T4 showed a positive correlation with peak serum GH (r=0.326, P<0.001). However, peak serum GH level after GH stimulation testing did not show a statistically significant association with age, IGF-1 SDS, or IGF-BP3 SDS. When the subjects were divided into GHD and ISS, both groups exhibited negative correlations of peak serum GH level in weight SDS (GHD: r=-0.312, P=0.033; ISS: r=-0.354, P=0.003), BMI SDS (GHD: r=-0.313, P=0.032; ISS: r=-0.246, P=0.043), and TSH (GHD: r=-0.299, P=0.046; ISS: r=-0.244, P=0.046) (Table 3). When we divided the subjects by subgroups of stimulants, BMI SDS demonstrated a significant correlation with peak GH in groups using dopamine (r=-0.419, P≤0.001), insulin (r=-0.271, P=0.044), and arginine (r=-0.368, P=0.038). Meanwhile, clonidine (r=0.071, P=0.880) and glucagon (r=0.186, P=0.447) did not exhibit a significant correlation.
4. Multiple regression analysis of BMI SDS and peak GH level after GH stimulation test
In univariate regression analysis, type of stimulants (P=0.042), BMI SDS (P<0.001), free T4 (P<0.001), and TSH (P=0.008) were associated with peak serum GH level after GH stimulation test. Type of stimulant was not a predictive factor of peak GH. In multiple regression analysis, BMI SDS (P=0.003) was negatively associated with peak serum GH level after GH stimulation test after adjusting for age, sex, pubertal status, and type of pharmacological stimulus. In the GHD group, BMI SDS (P=0.027) was negatively associated with peak serum GH level after GH stimulation testing. On the other hand, in the ISS group, there was no statistically significant association of peak serum GH level with BMI SDS (Table 4).
Discussion
The results of our study demonstrated that BMI SDS was a significant determinant of peak serum GH level after GH stimulation testing. BMI SDS, weight SDS, and free T4 level were significantly different between the GHD and ISS groups. In GH stimulation testing using dopamine, insulin, and arginine, peak serum GH level exhibited a negative correlation with weight SDS and BMI SDS. BMI SDS was the independent predictor of peak serum GH level in multiple regression analysis.
Previous studies have shown the relationship between obesity and GH secretion. It has been shown that adults with obesity have abnormal baseline GH secretion, and that obesity also is associated with impairment of GH response to GH stimulants [13-15]. These same results have been obtained for children. However, there are few short stature child patients who can be classified as overweight and obese compared to adults. In addition, standard values for pediatric BMI level differ by gender and age. Therefore, BMI SDS has been used to prove the effect of BMI on GH secretion in children. Several studies have reported on the relationship between BMI SDS and peak GH in children [16-20]. Although each study used a different number of subjects, different period of study, or different number of stimulants for analysis, they all demonstrated the common relationship between BMI SDS and peak serum GH.
Our results showed similar trends to previous studies about the effects of BMI on peak serum GH level. However, our study was different from previous studies in that we divided subjects into GHD and ISS groups and interpreted the effect of BMI SDS on peak serum GH level by group, separately. The results of our study showed that BMI SDS was associated with peak serum GH level in both GHD and ISS groups, and BMI SDS was an independent predictor of peak serum GH level in the total group and GHD group but not in the ISS group.
Since we could not diagnose GHD using levels of hormones like GH and IGF-1 with a single blood draw, confirmation of GHD relied on the GH response to GH stimulation test using 2 stimulants. However, the issue of reliability of GH stimulation testing has been continuously raised. Several studies [21-24] have reported that some patients diagnosed with isolated GHD have normal responses to stimulants when retested at the end of treatment or during treatment. This indicates that diagnosis of GHD could be a false positive. In this respect, the results of our study suggest that that BMI could be an important factor affecting GH responses to simulation testing in children with short stature, possibly leading to a false diagnosis of GHD. Therefore, more attention is needed to interpret the results of GH stimulation testing for a subject with higher BMI SDS.
In this study, we also divided subjects by stimulants and confirmed that BMI SDS exhibited a correlation with peak serum GH level when using dopamine, insulin, and arginine but not glucagon and clonidine. The mechanism of action of each stimulant is as follows. Dopamine acts on an alpha-adrenergic receptor of the central nervous system (CNS) and promotes the release of GHRH. The role of insulin in GH stimulation testing is to create a hypoglycemic state that could stimulate alpha-adrenergic receptors in the CNS and promote GHRH release from the hypothalamus. Glucagon and clonidine also act on the alpha-adrenergic system of the CNS to release GHRH from the hypothalamus and stimulate secretion of GH. The mechanism of arginine involves reduction in somatostatin release and induction of GH secretion [25]. In our study, BMI SDS exhibited a significant correlation with peak GH in groups using dopamine, insulin, and arginine as stimulants. These groups consisted of 30 or more subjects. We suggest that these results could be caused by the number of subjects. Dopamine, insulin, and arginine tests were performed with sufficient participants compared to the glucagon and clonidine groups. To analyze the cause of these results and determine the possible reasons, more research is needed with sufficient numbers of subjects.
The reason why obese people exhibit impaired GH secretion is not clear. However, high free fatty acid levels associated with actions of lipolysis [26,27] and elevated serum insulin actions on pituitary receptors that directly affect to suppression of GH secretion [28] were thought to be involved in the pathogenesis of insufficient GH secretion in obese patients. One more thing that needed to be analyzed in this study was the association of TSH with peak serum GH level. Studies on the positive correlation between obesity and TSH in euthyroid individuals have been performed. It is believed that obesity can influence the hypothalamus-pituitary-thyroid axis [29-33]. In our study, TSH exhibited a negative correlation with peak serum GH in both GHD and ISS groups. Although a few subjects in our study were overweight and obese, the relationship between BMI and TSH must be considered. Regarding the positive relationship between TSH and obesity and the negative correlation between obesity and peak serum GH level, the negative correlation of TSH with peak serum GH level could be explained.
There are several limitations in our study. First, since we used retrospective clinical data and researched with limited data, our sample size was not large enough to analyze each subgroup separately. Additional studies including a larger number of subjects must be conducted to compare results. Second, this is a retrospective, cross-sectional designed study, and we could not determine the causality of higher BMI and GHD. It also was difficult to exclude the possibility that patients with true GHD might have higher BMI, influencing our results. Despite several limitations, there are strengths of our study. Regardless of stimulant type, when a certain number of subjects was secured, BMI SDS is associated with peak GH. The test results of hormones that could affect growth and GHs, such as thyroid hormone, were analyzed together. In conclusion, our study confirmed that BMI could affect peak serum GH level. Therefore, we should consider BMI factors affecting peak GH when diagnosing GHD, especially for children with higher BMI.
Notes
Ethical statement
The study was approved by the Institutional Review Board of St. Vincent's Hospital of Catholic University (IRB No. VC19RESI0296).
Conflicts of interest
No potential conflict of interest relevant to this article was reported.