Introduction
The clinical findings of acquired hypothyroidism present insidiously with fatigue, constipation, decreased growth velocity, delayed bone age, compromised intellectual performance, obesity, myxedema, hyperlipidemia, peripheral neuropathy, and delayed or precocious puberty1,2). Small pericardial effusion is not an infrequent manifestation in primary hypothyroidism, but massive pericardial effusion is the uncommon complication of acquired hypothyroidism in children3). As cardiac tamponade is life-threatening hemodynamically, the prompt diagnosis and drainage of pericardial effusion should be performed. The most common and prominent manifestation of chronic acquired hypothyroidism in children is profound growth failure, which is severe and progressive4). Thyroxine supplementation improves all the clinical signs and symptoms except for growth failure5,6). Accelerated growth often does not result in restoration of full growth potential, due to the rapid increase in skeletal age during the first 18 months of treatment4).
This case report shows that autoimmune hypothyroidism in a prepubertal girl manifested as massive pericardial effusion, short stature, and hyperlipidemia. Even with thyroxine therapy, catch-up growth was incomplete by rapid increase in bone age. Growth hormone supplementation would improve restoration of the height deficit obtained during the hypothyroid state.
Case report
A 9-year-, 11-month-old girl with a history of sudden worsening dyspnea for one day was brought to the Emergency Department for one day. The previously healthy girl showed a weight increase of about 10 kg over the course of one year. However, there was no fatigue, constipation, or dry skin. Hypercholesterolemia was recently found in the school health examination, but no further work-up was performed. On examination, she was dyspneic but not cyanotic. Her vital signs were as follows; respiratory rate, 22 breaths/min; pulse rate, 65 beats/min; and blood pressure, 105/57 mmHg. Oxygen saturation (SaO2) was 100% in room air. She looked short but chubby, with a height of 120 cm (<3rd percentile, -2.61 height standard deviation score [HSDS]), weight 30 kg (25th percentile), and body mass index (BMI) 20.8 kg/m2 (90th-95th percentile). Her midparental height was 161 cm (0.05 HSDS). She had no goiter and was in the prepubertal state. On auscultation, heart sounds were soft and, distant, but there was no murmur.
The chest radiograph showed cardiomegaly with a waterbottle appearance. Sinus bradycardia with low voltages of the QRS complexes was noted in overall leads. On echocardiography, massive pericardial effusion around the heart was found, and fluctuating mitral inflow was recorded according to respiration (Fig. 1A). Complete blood count, electrolytes, renal function test, and urinalysis were normal. Serum total cholesterol was elevated at 526 mg/dL, low-density lipoprotein was 476 mg/dL, aspartate aminotransferase was 117 IU/L, alanine aminotransferase was 126 IU/L, and CPK (creatine phosphokinase) was 851 IU/L (normal, <167 IU/L). However, creatine kinase-myoglobin and troponin T were normal.
She was taken urgent pericardiocentesis, removing 110 mL of straw-colored fluid. Closed pericardiostomy was performed with a pericardial catheter (Fig. 1B), further draining 155 mL, and the catheter was removed two days later. The pericardial fluid showed that lactate dehydrogenase was 416 IU/L, glucose was 79 mg/dL, protein was 5.6 g/dL, and white blood cell count was 80/µL (mononuclear cell 90%, polymorphonuclear cell 10%), indicating exudate. The gram staining and culture for bacteria including acid-fast bacilli with the fluid were all negative. The polymerase chain reaction test was also negative for cytomegalic virus, Herpes simplex virus, and Epstein-Barr virus. An antibody test for Coxachie B virus was also negative.
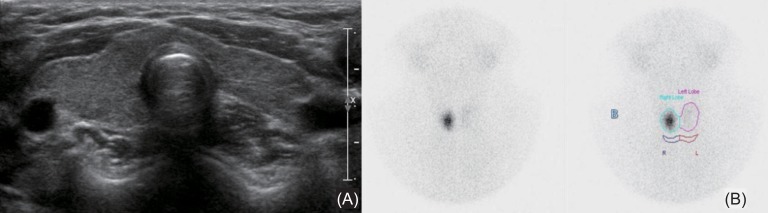
Endocrine tests were performed for short stature, high BMI, high cholesterol, relative bradycardia, and unknown etiology of pericardial effusion. Thyroid function analysis showed raised thyroid-stimulating hormone (TSH>50 µU/mL; normal range, 0.35-4.94 µU/mL) associated with a decrease of free T4 (0.27 ng/dL; normal range, 0.7-1.48 ng/dL) and T3 (56.9 ng/dL; normal range, 58.0-159 ng/dL). Thyroid autoantibodies, including antithyroglobulin antibody (154 IU/mL), thyroid peroxidase antibody (282 IU/mL), and TSH-receptor antibody (>40 U/L) were all positive, indicating autoimmune hypothyroidism. Bone age was 9.0 years, which showed a one year delay for chronologic age. Insulin-like growth factor (IGF)-1 and insulin-like growth factor binding protein (IGFBP)-3 levels were low, at 63.6 ng/mL (normal range at 9-10 years; 64-345 ng/mL) and 1,545 ng/mL (normal range at 9-10 years; 1,800-7,100 ng/mL), respectively. Thyroid ultrasound showed mild heterogeneous echogenicity without intrathyroid nodule and lymph node enlargement (Fig. 2A). Thyroid 99mTc scintigram showed a focal increase of the radio-uptake in the right lobe and nearly nonvisualization of the left lobe (Fig. 2B).
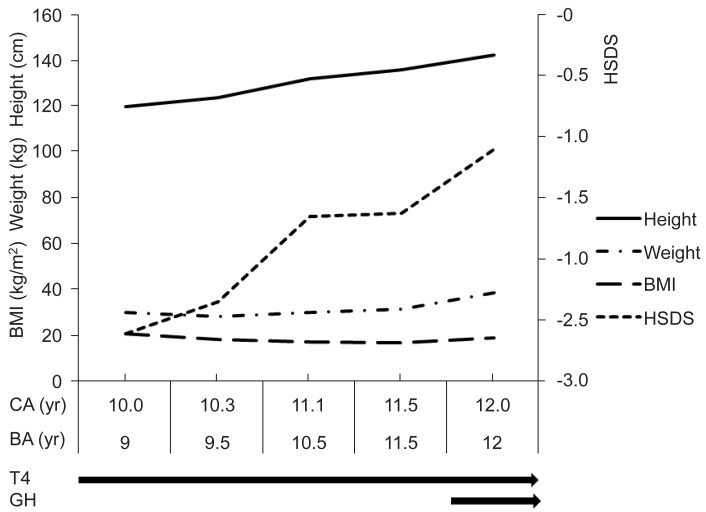
Thyroid hormone was replaced with 0.1 mg daily, and there was no recurrence of pericardial effusion since then. During the first year of thyroxine therapy, height velocity was 11.1 cm/yr, the HSDS improved from -2.61 to -1.65, and serum IGF-1 and IGFBP-3 levels were increased to 579 ng/mL and 2,687 ng/mL respectively. However bone age progressed rapidly from 9.0 years to 10.5 years. At 11 years of age, she developed breast budding with a bone age of 10.5 years. During the next 6 months, height velocity was 7.86 cm/yr and HSDS increased only to -1.62, but bone age progressed rapidly to 11.5 years. Growth hormone was given at 0.7 IU/kg/wk for 6 months, resulting in increased HSDS up to -1.10 at the end of the second year of treatment with a height velocity of 12.63 cm/yr (Fig. 3).
Discussion
This case is autoimmune hypothyroidism presenting as massive pericardial effusion with impending cardiac tamponade, which was demonstrated by the fluctuating mitral inflow of the Doppler pattern according to respiration. The relative bradycardia despite impending cardiac tamponade raised suspicion of a hypothyroid state. An endocrine test was performed to find the etiology of short stature, high BMI, and hypercholesterolemia. Clinical findings improved with thyroxine replacement, but catch-up growth was incomplete due to rapid skeletal maturation.
The incidence of newly diagnosed primary overt hypothyroidism among adults admitted through the Emergency Department is 0.1% in Taiwan. Most of the patients presented with nongoitrous autoimmune thyroiditis (21% of patients) and pericardial effusion (32% of patients) in the winter time7). Previously undiagnosed hypothyroidism can manifest as a myxedema coma with shock due to tamponade, particularly in the winter, because cold environments can precipitate myxedema8). Untreated long-standing hypothyroidism may evolve massive pericardial effusion in the case of child3). Our case also manifested massive effusion in December with nongoitrous autoimmune thyroiditis. The serous pericardial fluid may have cholesterol crystals with mononuclear cell infiltrates9). The mechanism of exudative pericardial effusion was thought to be a combination of the extravasation of albumin and inadequate lymphatic drainage10).
A thyroid function test should be performed in the presence of bradycardia with cardiac tamponade, and the absence of tachycardia in the presence of persistent hypotension11,12). In one report of the third recurrence of pericardial effusion, unexplained bradycardia, puffy face, and nonpitting pedal edema were clues for the diagnosis of hypothyroidism13). In our case, the clinical clues to suspect endocrine problems were short stature with high BMI, incidentally found hypercholesterolemia, and relative bradycardia in the presence of impending tamponade.
The low IGF-1 level in our case was a well-established phenomenon of decreased growth hormone secretion in severe hypothyroidism14), which increased with thyroxine therapy. The HSDS increased rapidly during the first year, but then HSDS slowly progressed during the next half year with rapid bone age progression. Case reports of prolonged primary hypothyroid girl with growth failure and delayed puberty showed incomplete recovery of growth potential with only thyroxine therapy5,6). The deficit in adult stature correlated with the duration of the hypothyroid state before treatment, so it is recommended to use a lower than usual replacement dosage of levothyroxine and/or to consider delaying puberty and epiphyseal fusion pharmacologically1). In other reports of the combined use of growth hormone and gonadotropin-releasing hormone agonist, the final height of two patients with Hashimoto's thyroiditis was improved15). However, a recent retrospective review suggested that neither time to euthyroidism nor the use of either gonadotropin-releasing hormone agonist or growth hormone significantly affected height potential16). We used short-term growth hormone with some success.
Our case had no goiter despite autoimmune hypothyroidism, which might be explained by thyroid parenchymal fibrosis with prolonged lymphocytic infiltration6), which was inferred from the nonvisualization of the left lobe in a 99mTc thyroid scintigram from our case.
In conclusion, primary hypothyroidism should be included in the etiologic evaluation of pericardial effusion, especially associated with relative bradycardia. Additional growth promoting therapy should be considered for incomplete catch-up growth in prolonged hypothyroidism during thyroxine supplementation.