 |
 |
- Search
| Ann Pediatr Endocrinol Metab > Volume 28(1); 2023 > Article |
|
Abstract
Supplementary Materials
Supplementary Fig. 1.
Notes
Fig. 1.

Fig. 2.
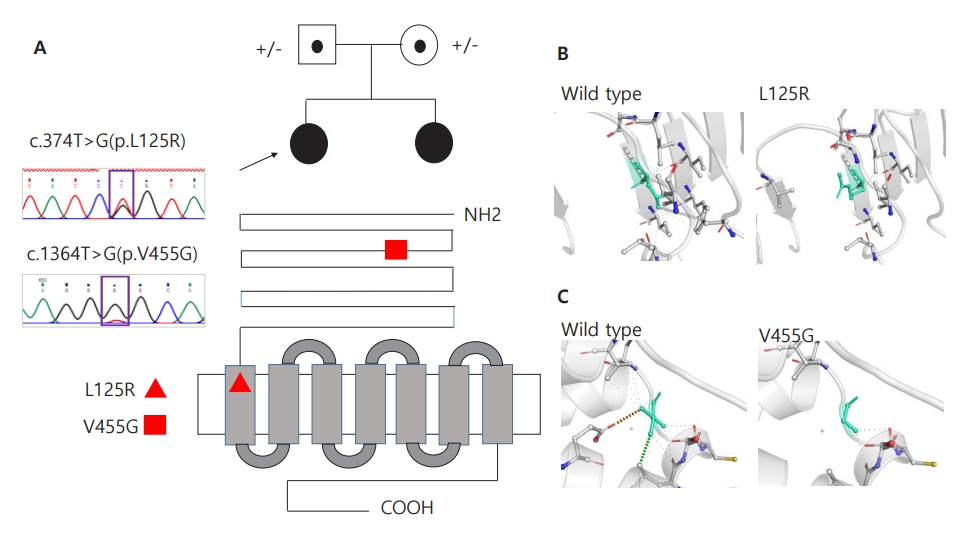
Table 1.
TSH, thyroid-stimulating hormone; ALP, alkaline phosphatase; LH, luteinizing hormone; FSH, follicle-stimulating hormone; SHBG, sex hormone binding globulin; DHEA-S, dehydroepiandrosterone sulfate; hCG, human chorionic gonadotropin; AMH, anti-Müllerian hormone; ACTH, adrenocorticotropic hormone; GnRH, gonadotropin-releasing hormone.
Table 2.
| Study | Zygosity | Exon | Genotype (Amino acid change) | Pathogenicity | Region | In vitro cell function† | I) Phenotype | Age‡ (yr) | Fertility |
|---|---|---|---|---|---|---|---|---|---|
| II) Reproductive structure | |||||||||
| III) Follicular development | |||||||||
| Present study (2020) | COM | 4 | c.374T>G (p.Leu125Arg) | ND | ECD | ND | I) Hypergonadotropic primary amenorrhea | 16 | NA |
| 10 | c.1364T>G (p.Val455Gly) | TMD | II) Normal sized ovaries. Bicornuate uterus (patient 1). Hypoplastic uterus with endometrial thinning (patient 2) | 19 | NA | ||||
| França et al. [15] (2017) | HOM | 10 | c.1298C>A (p.Ala433Asp) | ND | EL1 | ND | I) Hypergonadotropic primary amenorrhea | 15 | NA |
| II) Small bilateral ovaries and a small uterus on US | |||||||||
| Liu et al. [16] (2017) | HOM | 2 | c.175C>T (p.Arg59Term) | U | ECD | A | I) Hypergonadotropic primary amenorrhea | 30 | NA |
| II) Hypoplastic uterus, 2 small gray ovaries without any | |||||||||
| III) Follicular arrest – small antral stage | |||||||||
| Bramble et al. [17] (2016) | HOM | 10 | c.1222G>T (p.Asp408Tyr) | ND | TMD | A+C | I) Hypergonadotropic primary amenorrhea | 22 | NA |
| II) Small bilateral ovaries and a small uterus on US | |||||||||
| III) no visible antral follicle | |||||||||
| Katari et al. [18] (2015) | HOM | 10 | c.1253T>G (p.Ile418Ser) | ND | TMD | ND | I) Hypergonadotropic primary amenorrhea | 14 | NA |
| II) A single small right ovary and a small uterus on US | |||||||||
| Achrekar et al. [19] (2010) | HOM | 10 | c.1723C>T (p.Ala575Val) | LP | TMD | ND | I) Hypergonadotropic primary amenorrhea | 16 | NA |
| II) Two very small ovaries and a hypoplastic uterus with thin endometrium | |||||||||
| Kuechler et al. [20] (2010) | HET | 10 | c.1760C>A (p.Pro587His) | P/LP | TMD | A | I) Hypergonadotropic primary amenorrhea | 17 | NA |
| II) Normal sized ovaries and a small uterus on diagnostic laparoscopy | |||||||||
| III) Disturbed folliculogenesis with a high number of primordial follicle | |||||||||
| Nakamura et al. [21] (2008) | HOM | 8 | c.662T>G (p.Val221Gly) | LP | ECD | ND | I) Hypergonadotropic primary amenorrhea | 25 | No |
| II) Normal sized ovaries | |||||||||
| III) Follicular arrest – small antral stage | |||||||||
| Allen et al. [22] (2003) | HET | 10 | c.1043C>G (p.Pro348Arg) | LP | ECD | A+C | I) Hypergonadotropic primary amenorrhea | 17 | NA |
| II) Normal but relatively immature Müllerian duct-derived structures and bilateral streak ovaries on diagnostic laparoscopy | |||||||||
| Meduri et al. [23] (2003) | HOM | 10 | c.1555C>A (p.Pro519Thr) | P | EL2 | A+B+C | I) Hypergonadotropic primary amenorrhea | 26 | NA |
| II) Two hypoplastic ovaries and a small uterus on US | |||||||||
| III) Follicular arrest – small antral stage | |||||||||
| Doherty et al. [24] (2002) | COM | 7 | c.566C>T (p.Ala189Val) | P | ECD | A+B+C | I) Hypergonadotropic primary amenorrhea | 17 | NA |
| 10 | c.1255G>A (p.Ala419Thr) | P | TMD | A | |||||
| Touraine et al. [25] (1999) | COM | 7 | c.671A>T (p.Asp224Val) | LP | ECD | A+B+C | I) Hypergonadotropic primary amenorrhea | 19 | NA |
| 10 | c.1801C>G (p.Leu601Val) | LP | EL3 | A | II) Normal sized ovaries and a uterus on US | ||||
| III) Follicular arrest – small antral stage | |||||||||
| Beau et al. [26] (1998) | COM | 6 | c.479T>C (p.Ile160Thr) | CIP | ECD | A+B+C | I) Secondary amenorrhea with increased serum FSH levels | 30 | No |
| 10 | c.1717C>T (p.Arg573Cys) | P | IL3 | A | II) Normal sized ovaries and a small uterus on US | ||||
| III) Follicular arrest – small antral stage | |||||||||
| Gromoll et al. [27] (1996) | HET | 7 | c.573A>T (p.Asn191Ile) | ND | ECD | A | I) Fertile woman with normal ovarian function | NA | Yes |
| Aittomäki et al. [28] (1995) | HOM | 7 | c.566C>T (p.Ala189Val) | P | ECD | A+B+C | I) Hypergonadotropic primary amenorrhea | NA | NA |
| II) Ovarian dysgenesis |
Table 2 is written in reference to study of He et al. [6]
CIP, variants with conflicting interpretations of pathogenicity; COM, compound heterozygote; ECD, extracellular ligand binding domain; EL, extracellular loop; HET, heterozygote; HOM, homozygote; IL, intracellular; LP, likely pathogenic; LTD, intervening loop of transmembrane domain; MTD, membrane-spanning region of transmembrane domain; NA, not available; ND, not determined; P, pathogenic; TMD, transmembrane domain; U, uncertain significance; US, ultrasonography.
References
- TOOLS
- Related articles in APEM







