1. LaFranchi SH. Newborn screening strategies for congenital hypothyroidism: an update. J Inherit Metab Dis 2010;33(Suppl 2):S225–S233. PMID:
20195902.


2. LaFranchi SH. Approach to the diagnosis and treatment of neonatal hypothyroidism. J Clin Endocrinol Metab 2011;96:2959–2967. PMID:
21976744.


3. Lanting CI, van Tijn DA, Loeber JG, Vulsma T, de Vijlder JJ, Verkerk PH. Clinical effectiveness and cost-effectiveness of the use of the thyroxine/thyroxine-binding globulin ratio to detect congenital hypothyroidism of thyroidal and central origin in a neonatal screening program. Pediatrics 2005;116:168–173. PMID:
15995048.


4. Fujiwara F, Fujikura K, Okuhara K, Tsubaki J, Fukushi M, Fujita K, et al. Central congenital hypothyroidism detected by neonatal screening in Sapporo, Japan (2000-2004): it\'s prevalence and clinical characteristics. Clin Pediatr Endocrinol 2008;17:65–69. PMID:
24790365.



5. Tajima T, Jo W, Fujikura K, Fukushi M, Fujieda K. Elevated free thyroxine levels detected by a neonatal screening system. Pediatr Res 2009;66:312–316. PMID:
19542904.


6. Adachi M, Soneda A, Asakura Y, Muroya K, Yamagami Y, Hirahara F. Mass screening of newborns for congenital hypothyroidism of central origin by free thyroxine measurement of blood samples on filter paper. Eur J Endocrinol 2012;166:829–838. PMID:
22301913.


7. Kelberman D, Dattani MT. Role of transcription factors in midline central nervous system and pituitary defects. Endocr Dev 2009;14:67–82. PMID:
19293576.


8. Pfäffle R, Klammt J. Pituitary transcription factors in the aetiology of combined pituitary hormone deficiency. Best Pract Res Clin Endocrinol Metab 2011;25:43–60. PMID:
21396574.


9. Tajima T, Ishizu K, Nakamura A. Molecular and clinical findings in patients with LHX4 and OTX2 Mutations. Clin Pediatr Endocrinol 2013;22:15–23. PMID:
23990694.



10. Miyai K, Azukizawa M, Kumahara Y. Familial isolated thyrotropin deficiency with cretinism. N Engl J Med 1971;285:1043–1048. PMID:
4106196.


11. Hayashizaki Y, Hiraoka Y, Endo Y, Miyai K, Matsubara K. Thyroid-stimulating hormone (TSH) deficiency caused by a single base substitution in the CAGYC region of the beta-subunit. EMBO J 1989;8:2291–2296. PMID:
2792087.



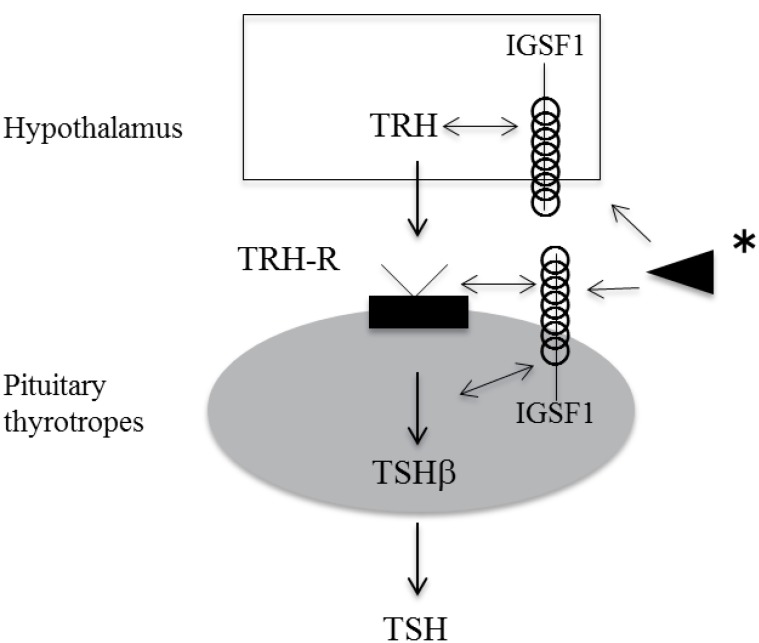
12. Collu R, Tang J, Castagne J, Lagace G, Masson N, Huot C, et al. A novel mechanism for isolated central hypothyroidism: inactivating mutations in the thyrotropin-releasing hormone receptor gene. J Clin Endocrinol Metab 1997;82:1561–1565. PMID:
9141550.


13. Bonomi M, Busnelli M, Beck-Peccoz P, Costanzo D, Antonica F, Dolci C, et al. A family with complete resistance to thyrotropin-releasing hormone. N Engl J Med 2009;360:731–734. PMID:
19213692.

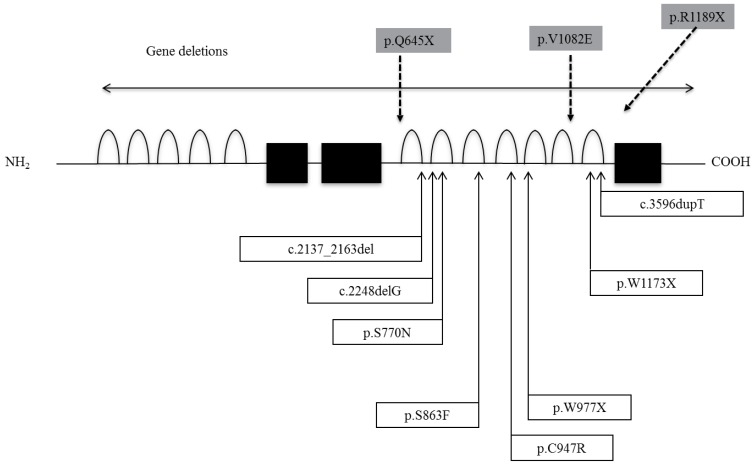
14. Sun Y, Bak B, Schoenmakers N, van Trotsenburg AS, Oostdijk W, Voshol P, et al. Loss-of-function mutations in IGSF1 cause an X-linked syndrome of central hypothyroidism and testicular enlargement. Nat Genet 2012;44:1375–1381. PMID:
23143598.



15. Tajima T, Nakamura A, Ishizu K. A novel mutation of IGSF1 in a Japanese patient of congenital central hypothyroidism without macroorchidism. Endocr J 2013;60:245–249. PMID:
23363888.


16. Nakamura A, Bak B, Silander TL, Lam J, Hotsubo T, Yorifuji T, et al. Three novel IGSF1 mutations in four Japanese patients with X-linked congenital central hypothyroidism. J Clin Endocrinol Metab 2013;98:E1682–E1691. PMID:
23966245.


17. Joustra SD, Schoenmakers N, Persani L, Campi I, Bonomi M, Radetti G, et al. The IGSF1 deficiency syndrome: characteristics of male and female patients. J Clin Endocrinol Metab 2013;98:4942–4952. PMID:
24108313.


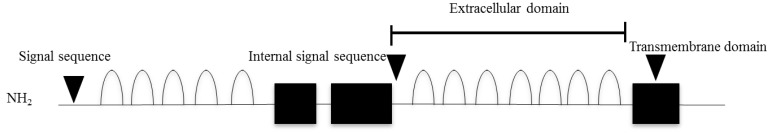
18. Mazzarella R, Pengue G, Jones J, Jones C, Schlessinger D. Cloning and expression of an immunoglobulin superfamily gene (IGSF1) in Xq25. Genomics 1998;48:157–162. PMID:
9521868.


19. Robakis T, Bak B, Lin SH, Bernard DJ, Scheiffele P. An internal signal sequence directs intramembrane proteolysis of a cellular immunoglobulin domain protein. J Biol Chem 2008;283:36369–36376. PMID:
18981173.



20. Bernard DJ, Chapman SC, Woodruff TK. Inhibin binding protein (InhBP/p120), betaglycan, and the continuing search for the inhibin receptor. Mol Endocrinol 2002;16:207–212. PMID:
11818494.


21. Bernard DJ, Burns KH, Haupt B, Matzuk MM, Woodruff TK. Normal reproductive function in InhBP/p120-deficient mice. Mol Cell Biol 2003;23:4882–4891. PMID:
12832474.



22. Chapman SC, Bernard DJ, Jelen J, Woodruff TK. Properties of inhibin binding to betaglycan, InhBP/p120 and the activin type II receptors. Mol Cell Endocrinol 2002;196:79–93. PMID:
12385827.


23. Barclay AN. Membrane proteins with immunoglobulin-like domains: a master superfamily of interaction molecules. Semin Immunol 2003;15:215–223. PMID:
14690046.


24. Xu Z, Jin B. A novel interface consisting of homologous immunoglobulin superfamily members with multiple functions. Cell Mol Immunol 2010;7:11–19. PMID:
20081873.



25. Ohtani H, Nakajima T, Akari H, Ishida T, Kimura A. Molecular evolution of immunoglobulin superfamily genes in primates. Immunogenetics 2011;63:417–428. PMID:
21390552.


26. Babu K, Hu Z, Chien SC, Garriga G, Kaplan JM. The immunoglobulin super family protein RIG-3 prevents synaptic potentiation and regulates Wnt signaling. Neuron 2011;71:103–116. PMID:
21745641.



27. Irintchev A, Schachner M. The injured and regenerating nervous system: immunoglobulin superfamily members as key players. Neuroscientist 2012;18:452–466. PMID:
21903634.


28. Yu XM, Gutman I, Mosca TJ, Iram T, Ozkan E, Garcia KC, et al. Plum, an immunoglobulin superfamily protein, regulates axon pruning by facilitating TGF-β signaling. Neuron 2013;78:456–468. PMID:
23664613.



29. Malaguti A, Della Casa C, Castorina S, Martelli AM, Roti E, Martino E, et al. Molecular mechanisms for pituitary thyrotroph cell growth. J Endocrinol Invest 2004;27(6 Suppl):151–167. PMID:
15481817.

30. Shibusawa N, Yamada M, Hirato J, Monden T, Satoh T, Mori M. Requirement of thyrotropin-releasing hormone for the postnatal functions of pituitary thyrotrophs: ontogeny study of congenital tertiary hypothyroidism in mice. Mol Endocrinol 2000;14:137–146. PMID:
10628753.


31. Inokuchi M, Matsuo N, Takayama JI, Hasegawa T. Tracking of BMI in Japanese children from 6 to 18 years of age: reference values for annual BMI incremental change and proposal for size of increment indicative of risk for obesity. Ann Hum Biol 2011;38:146–149. PMID:
20632778.