Hypertriglyceridemia with acute pancreatitis in a 14-year-old girl with diabetic ketoacidosis
Article information
Abstract
Diabetic ketoacidosis (DKA) is a medically fatal condition in poorly controlled hyperglycemia or newly diagnosed diabetes mellitus. Severe hypertriglyceridemia (HTG) is an uncommon complication of DKA and can be associated with acute pancreatitis (AP). We present the clinical manifestations, laboratory findings, and management of AP associated with HTG in a 14-year-old girl with DKA. The patient, with a 7-year history of type 2 diabetes presented with epigastric pain, 1 month after stopping insulin injection. DKA, severe HTG, and AP were diagnosed based on the laboratory and imaging tests. She recovered from DKA after conventional treatment for DKA, and her triglyceride (TG) level was reduced from 10,867 mg/dL to the normal range after 7 days of admission without antilipid medication. Given that her C-peptide level was not too low and considering her negative diabetes-related antibodies and high TG level, targeted gene panel sequencing was performed on the genes associated with diabetes and HTG. We identified a heterozygous mutation, c.4607C>T (p. Ala1537Val), in ABCC8 related to maturityonset diabetes of the young (MODY) 12. To our knowledge, this is the first reported case of HTG-induced AP with DKA in a patient with MODY. In addition, we reviewed the literature for pediatric cases of HTG with DKA. In patients with DKA, timely awareness of severe HTG related to insulin deficiency is crucial for improving the consequences of AP. We recommend considering AP in all DKA patients presenting with severe HTG to ensure early and proper management.
Highlights
Severe HTG is an uncommon complication of DKA and can be associated with AP. In patients with DKA, timely awareness of severe HTG related to insulin deficiency is crucial for improving the consequences of AP.
Introduction
Diabetic ketoacidosis (DKA) is a medically fatal condition that may occur in patients with poorly controlled hyperglycemia or newly diagnosed diabetes mellitus (DM). Cerebral edema is its most devastating complication and causes >20% of deaths among DKA cases [1]. Severe hypertriglyceridemia (HTG, triglyceride [TG] > 1,000 mg/dL) is an uncommon complication of DKA and can be associated with acute pancreatitis (AP) [2]. The combination of DKA, HTG, and AP has been discussed in adults. While severe HTG was identified in around 8% of adult DKA cases, data of this combination in children have remained limited [3,4]. Here, we present the clinical manifestations, laboratory findings, and management of AP associated with HTG in a 14-year-old girl with DKA. In addition, literature on HTG with DKA in pediatric cases was reviewed.
Case Report
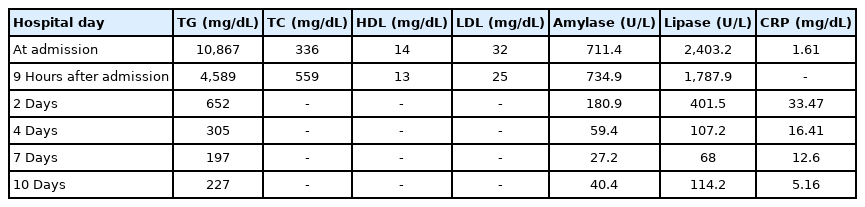
A 14-year-old girl presented to Samsung Medical Center owing to severe epigastric pain, nausea, and fever for 1 day. She was diagnosed with type 2 DM at another hospital at the age of 7 years and 10 months, when her hemoglobin A1c (HbA1c) was 10.1% and postprandial C-peptide level was 7.2 ng/mL, while her diabetes-related antibodies were all negative. She was treated with metformin (≤2 g/day) in the early stage of DM; however, basal insulin (glargine) was added to metformin owing to the poor control of hyperglycemia (HbA1c level, 14.0%). It was 1 month before this episode that she discontinued blood glucose testing and regular insulin injections. When she presented to our hospital, her mental status was alert and vital signs included a blood pressure of 132/70 mmHg, heart rate of 157 bpm, respiratory rate of 24 breaths/min, and body temperature of 38.2℃. Her body weight, height, and body mass index were 60.1 kg (standard deviation score [SDS], 1.18), 157.4 cm (SDS, -0.07), and 24.3 kg/m2 (SDS, 1.38), respectively. She showed a dry mouth and decreased skin turgor. Her abdomen was soft and distended, and the bowel sounds were normal. She complained of tender epigastrium on palpation. Her laborator y findings were suggestive of DKA, such as a glucose level of 311 mg/dL, venous blood gas with a pH of 7.2, pCO2 of 21 mmHg, HCO3− of 8.2 mmol/L, base excess of -17.8 mmol/L, and β-hydroxybutyrate level of 3.4 mmol/L (normal reference < 0.4–0.5 mmol/L). Her HbA1c level was 14.2%, and urinalysis revealed 3+ glucose and 3+ ketones. Other laboratory findings included the following results: white blood cell count of 12,380/μL, hemoglobin level of 13.5 g/dL, platelet count of 205,000/μL, total cholesterol level of 336 mg/dL, high-density lipoprotein (HDL) level of 14 mg/dL, TG level of 10,867 mg/dL, low-density lipoprotein (LDL) level of 32 mg/dL, aspartate aminotransferase level of 16 U/L, alanine aminotransferase level of 19 U/L, amylase level of 711.4 U/L (normal reference, 28–100 U/L), lipase level of 2,403.2 U/ L (normal reference, 13–60 U/L), sodium level of 133 mmol/ L, potassium level of 3.9 mmol/L, chloride level of 97 mmol/L, and C-reactive protein (CRP) level of 1.61 mg/dL. Antiglutamic acid decarboxylase antibody and anti-insulin auto-antibody tests were negative. Eruptive xanthoma or xanthelasma was not observed. There were no abnormalities, such as lipemia retinalis, found on an ophthalmologic examination. Abdominal computed tomography (CT) imaging revealed a diffuse edematous pancreas with adjacent fluid collection, which suggested AP of grade D (Fig. 1A). In addition, she had a fatty liver. No gallbladder involvement was seen.
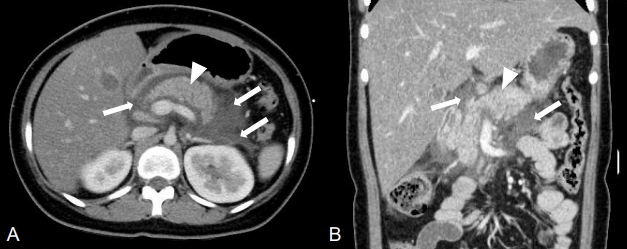
Contrast-enhanced abdominal computed tomography (CT) imaging. The initial CT scan shows edematous pancreas (arrowhead) and adjacent fluid collection (arrows), suggesting grade D acute pancreatitis. (A) Axial image, (B) coronal image.
Immediate management included intravenous rehydration therapy, continuous intravenous insulin infusion (6 units/hr), experimental antibiotics (piperacillin and tazobactam) for AP, and an analgesic (1 g of propacetamol) for pain control. On the second day of admission, her CRP level had increased to 33.47 mg/dL and the abdominal pain had persisted. She did not complain of steatorrhea. Follow-up CT imaging of the abdomen showed an increased volume of peripancreatic fluid collection relative to the previous examination suggesting AP of grade D (Fig. 1B). Her antibiotics were changed to broadspectrum antibiotics (meropenem and vancomycin). Four days after admission, her TG level had declined to 305 mg/dL, and her CRP level was 16.41 mg/dL. The abdominal pain had also resolved. Her blood culture was negative, and antibiotics were changed to piperacillin and tazobactam. She recovered from DKA, and her TG level decreased to 197 mg/dL without antilipid medication 7 days after admission. Because her abdominal pain had resolved and the serum amylase and lipase were nearly normal (27.2 and 68 U/L, respectively), she commenced with a normal diet. Repeated ultrasonography showed resolution of pancreatic inflammation. She did not have complications of diabetes, including retinopathy, nephropathy, or neuropathy. Serial laboratory results related to HTG and AP during hospitalization are summarized in Table 1.
Given that her C-peptide level was not too low and considering her negative diabetes-related antibodies and high TG level, targeted gene panel sequencing was performed on the genes associated with diabetes and HTG. With informed consent from the patient, DNA was isolated from the peripheral blood leukocyte using the chemagic Magnetic Separation Module I method (PerkinElmer, Waltham, MA, USA). In total, 57,000 target exons of a total of 4,503 clinically relevant genes were captured by the xGen Inherited Disease Panel (Integrated DNA Technologies, Inc., Coralville, IA, USA) and sequenced with the NextSeq500 platform (Illumina, San Diego, CA, USA) for 2 × 150-bp paired-end reads, which were mapped to the hg19 utilizing the Burrow-Wheeler Aligner (ver. 0.7.12). For local realignment, recalibration, and variant-calling, the Genome Analysis Tool Kit (ver. 3.5) was used. We identified a heterozygous variant, c.4607C>T (p. Ala1537Val), in exon 38 of the ATP-binding cassette transporter subfamily C member 8 (ABCC8) for maturity-onset diabetes of the young (MODY) 12, and no pathogenic variant was detected in other genes. The missense variant c.4607C>T (p.Ala1537Val) is considered a variant of unknown significance (VUS) based on the American College Medical Genetics and Genomics guidelines. This variant was found at a frequency of 0.0008% in a population database (gnomAD) and classified as being of "uncertain significance" in ClinVar. This variant was predicted to be "damaging" by algorithms developed to predict the effect of missense changes on protein structure (SIFT, Polyphen2, and MutationTaster). Generally, family segregation analysis can determine the pathogenicity of a VUS, but our patient’s family members were not readily available for genetic testing and clinical information. After discharge, she was instructed to take basal insulin before breakfast and insulin aspart 3 times daily before meals.
Discussion
This case emphasizes the necessity for the recognition of AP associated with severe HTG in patients with DKA. DKA represents a state of severe insulin deficiency characterized by hyperglycemia and metabolic acidosis with ketone accumulations [5]. The clinical presentations and complications of DKA result from hyperglycemia, dehydration, ketosis, and electrolyte imbalance. HTG in DKA is attributed to insulin deficiency, leading to increased lipolysis. Consequently, free fatty acid (FFA) is released, which activates the synthesis of very-LDL (VLDL) [6,7]. Lipoprotein lipase (LPL) is responsible for the removal of VLDL and chylomicrons from the bloodstream. Insulin deficiency leads to decreased LPL activity, which causes HTG [8]. Severe HTG is rare; however, it is an important risk factor for AP [2]. Pancreatic lipase hydrolyzes TG, inducing the production of FFA, which activates trypsinogen and causes autodigestion of the pancreatic gland [9]. Major treatments of severe HTG include insulin, heparin, and plasmapheresis; however, few studies have enrolled children and adolescents to date. Maintaining a TG level of <500 mg/dL is believed to result in symptom improvement. Insulin activates LPL, facilitating the degradation and clearing of TG. Heparin leads to the release of LPL from the endothelial cells, resulting in TG degradation. Oral antilipid medications are recommended when patients are capable of an oral diet, and fibrates can lower TG levels by 40%–60% [10]. In routine practice, the Friedewald equation is used to estimate LDL (total cholesterol [mg/dL] − HDL [mg/dL] − TG [mg/dL]/5). It has been believed that LDL is not exactly estimated when TG level exceeds 400 mg/dL [11]. Generally, the Friedewald equation works properly in normolipidemia, not hyperlipidemia. Thus, there is a discrepancy between the LDL estimated by the equation and the actual measurements in our case. Published pediatric cases of AP associated with HTG in DKA are summarized in Table 2. All reports showed that severe HTG is an identifiable risk factor for AP [2]. TG levels gradually decreased to <500 mg/dL 1–14 days after management. In our patient, hydration and insulin infusion resulted in resolution of DKA and concurrent normalization of HTG. Initial lipid profiling is important in patients with DKA, because it can be a clue for timely abdominal imaging to ensure a diagnosis of AP. Moreover, abdominal pain is a common presentation of DKA as well as AP, with the symptom reported in nearly 50% of cases and related to variable gastrointestinal manifestations [12].
The ABCC8 gene, encoding the sulfonylurea receptor 1 subunit of the ATP-sensitive potassium (KATP) channel, can regulate the secretion of insulin. ABCC8 mutations have been shown to cause congenital hyperinsulinism (CHI), type 2 DM, gestational DM, neonatal diabetes, and MODY [13]. CHI is associated with the blindness of pancreatic β-cells responsible for insulin secretion, causing severe and persistent hypoglycemia. CHI is classified histologically into diffuse hyperinsulinism or focal islet-cell hyperplasia. Diffuse hyperinsulinism appears in an autosomal recessive manner, and entire β-cells in the pancreas are affected. On the other hand, its focal form is a heterozygous paternally inherited KATP mutation of chromosome 11p15 region, which is confined to the islet cells of focal lesions [14]. It is challenging to differentiate MODY from other types of diabetes depending on clinical manifestations. In patients with MODY, β-cell function is generally conserved, and insulin is not required in the early stage of the disease [15]. Kapoor et al. [16] reported a dominant ABCC8 mutation, A1537V, identified in our patient, which causes an asymptomatic carrier, hyperinsulinemic hypoglycemia, and gestational DM within 3 generations of a single family. Mutations in ABCC8 can be associated with both hyperactivity and underactivity of the KATP channel. Slow and progressive damage to β-cells owing to increased β-cell apoptosis can lead to both remission of hyperinsulinism and progression to diabetes [17]. Considering the clinical variability of ABCC8 mutations, the mutation identified in our patient is suspected to be related to MODY 12. As the result of target gene panel sequencing was classified as a VUS, family segregation analysis can enforce the pathogenicity of this variant. However, it was not possible to collect the detailed family history and conduct genetic testing of relatives in this case. This is the limitation of our case and thus, further validations in additional patients and functional studies are needed to prove the pathogenicity of this variant. Patients with MODY 12 respond to sulfonylurea therapy [13]. Thus, switching our patient from insulin to sulfonylurea is under consideration.
In this study, we presented a 14-year-old girl with AP related to severe HTG in DKA. To our knowledge, this is the first report of HTG-induced AP with DKA in a patient with MODY. In patients with DKA, timely awareness of severe HTG related to insulin deficiency is crucial for improving the consequence of AP. Based on our experience and the review of pertinent literature, we recommend considering AP in all DKA patients presenting with severe HTG to ensure early and proper management.
Notes
Ethical statement
Informed consent was obtained from the parents of the patient.
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Funding
This study was supported by a grant from Samsung Medical Center (#GFO3200061).
Author contribution
Conceptualization: HP, SYC, EGY; Data curation: HP, EGY; Formal analysis: MSK, SML; Project administration: SYC, DKJ; Visualization: JK; Writing - original draft: HP; Writing - review & editing: HP