Update on the current modalities used to screen high risk youth for prediabetes and/or type 2 diabetes mellitus
Article information
Abstract
The modalities currently employed to screen for type 2 diabetes mellitus (T2DM)/prediabetes are HbA1c, fasting plasma glucose (FPG), and 2-hour plasma glucose (PG) during an oral glucose tolerance test (OGTT). The purpose of this review is to highlight the positive qualities and pitfalls of these diagnostic modalities and reflect on the most reasonable and effective approach to screen high risk youth. Given its inherent preanalytical advantages, glycated hemoglobin (HbA1c) continues to be the preferred diagnostic modality used by pediatricians to screen high risk youth. However, when the three aforementioned tests are performed in youths of different races/ethnicities, discrepant results for T2DM/prediabetes are observed. The prevalence rates for T2DM vary from 0.53% in Chinese youth (including youth of all body mass indexes) to 18.3% in high-risk, overweight, obese Korean youth. Moreover, the FPG is abnormal (>100 less than <126 mg/dL) in 15% of Korean youth versus 8.7% of Chinese youth. The prevalence rates for prediabetes are 1.49% in Chinese youth versus 21% in Emirati youth (HbA1c, 5.7%–6.4%). The coefficient of agreement, k, between these screening tests for T2DM are fair, 0.45–0.5 across all youth. However, using HbA1c as a comparator, the agreement is weak with FPG (k=0.18 in German youth versus k=0.396 in Korean youth). The American Diabetes Association (ADA) Standards of Medical Care Guidelines define “high risk youth” who need to be tested for T2DM and/or prediabetes. OGTT and HbA1c do not always detect T2DM in similar individuals. HbA1c may not be an ideal test for screening Hispanic and African American youth. FPG and OGTT are suitable screening tests for youth of ethnic minorities and those with cystic fibrosis or hemoglobinopathies. Performing all three tests either together or sequentially may be the only way to encompass all youth who have aberrations in different aspects of glucose homeostasis.
Introduction
Prediabetes, a condition in which the glucose levels are not high enough to meet the criteria of diabetes but also cannot be considered normal, is an intermediate state along the spectrum of glucose homeostasis.
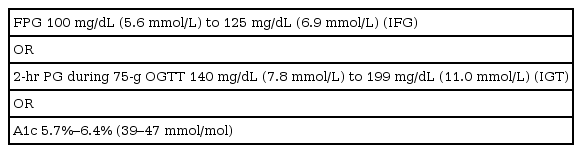
Table 1 defines type 2 and prediabetes as per the Standard of Medical Care in Diabetes update in 2019 [1]. In 2010, the ADA added glycated hemoglobin (HbA1c) of ≥6.5% as diagnostic criteria for type 2 diabetes mellitus (T2DM) and made HbA1c of 5.7%–6.4% (39–47 mmol/mol) as the criteria for prediabetes [1]. The risk for developing diabetes is a continuum and spans from low below the cut-off values to disproportionately higher at the upper end of this range.
Prevalence of type 2/prediabetes in youth
The obesity epidemic has led to an exponential increase in prediabetes and/or mellitus (T2DM, and this rise has been the most dramatic in Hispanic, American-Indian and African-Americans populations [2,3]. Thirty years ago, T2DM in youth was considered rare; the first cases of T2DM were reported in the mid-1990s [4]. However, the last decade has seen a dramatic increase in the numbers of youth with T2DM, especially in racial and ethnic minorities [2], and projections suggest that there will be 1,000,000 youths with T2DM in the United States by the year 2050 [5].
The prevalence rates for prediabetes in youth are highly variable and range from 18%–28% in obese youth [6]. The conversion rate of prediabetes to T2DM is accelerated in youth, with studies showing a 15% annual reduction in beta-cell function with a mean transition time from prediabetes to diabetes of 2.5 years more so than in adults [7]. Therefore, in high risk youth with prediabetes, screening, diagnosis, and therapeutic lifestyle counselling are paramount for diabetes prevention.
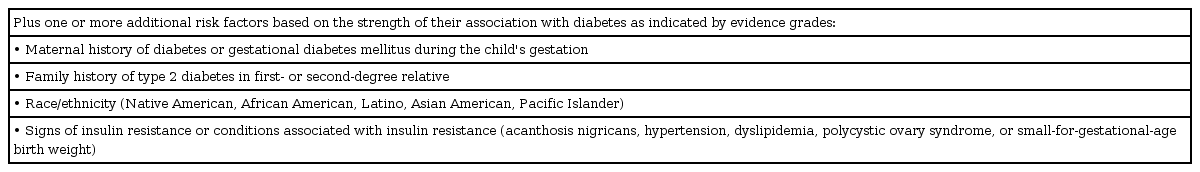
Table 2 defines the risk-based screening criteria in order to provide guidance to pediatricians for initial and follow-up diabetes screening in high risk youth [8]. The value of the diagnostic tools approved by the ADA have been adopted to help identify asymptomatic youth at an increased risk for T2DM and/or prediabetes.
FPG and 2-hour PG as diagnostic tools
Historically, the diagnostic thresholds for impaired fasting glucose (IFG) and 2-hour plasma glucose (PG) were established from epidemiologic studies in adults, which found that the diabetes-related complication of retinopathy was prevalent and increased exponentially above the fasting PG cut off of 126 mg/dL and the 2-hour PG cutoff of 200 mg/dL, thus defining these thresholds [9]. The World Health Organization defines IFG as 110 mg/dL (6.1 mmol/L).
The oral glucose tolerance test (OGTT) diagnostic criteria have been considered the "gold standard" since their inception in 1997. Fasting plasma glucose (FPG) and 2-hour PG cut off values have not been validated in youth, and studies to do so are unlikely.
Benefits and pitfalls of FPG and 2-hour OGTT
The OGTT provides an overview of different aspects of beta cell function (fasting and prandial) within the background of insulin resistance, which is present in most obese youth. Moreover, it can illustrate whether there is more hepatic insulin resistance (higher FPG) versus postprandial resistance (higher 2-hour PG).
The main disadvantages of the OGTT are as follows: (1) the requirement for an overnight fast of at least 8 hours, which could be a challenge for young children; (2) difficulty in staffing pediatric offices to conduct the OGTT during office hours; (3) the length of the test (2 hours) can be a deterrent for both families and physicians; and (4) Some pediatricians do not feel comfortable ordering or interpreting OGTT results. The major caveat of OGTT in pediatrics is that it has poor reproducibility, and there have been no validation studies in youth [10]. A study at Yale in youths with prediabetes who were followed with serial OGTTs revealed that 50% of youths with prediabetes reverted to normal glucose tolerance, whereas 24% progressed from prediabetes to diabetes [11].
Below an HbA1c level of 7%, 2-hour PG reflects prandial hyperglycemia increases before increases of FPG, whereas in patients with an HbA1c level above 8%, the contribution of basal hyperglycemia to overall hyperglycemia becomes predominant. So, youth with impaired glucose tolerance may have near-normal HbA1c, and these youth will be missed if only FPG is done without an OGTT.
Although FPG requires fasting, its advantage is that it requires only one blood draw. FPG is affected by stress/illness and drops by 5%–7% per hour in a sample due to glycolysis. Both FPG and OGTT have intra-individual variability, which is much higher for OGTT at 12.7% versus 5.7%–8.3% for FPG [12].
HbA1c as a diagnostic tool
The HbA1c test was used in prospective studies in adults, and it was found to have a consistent linear association with the development of diabetes. Systematic reviews of over 40,000 adults from 16 studies have shown that the 5-year risk for developing diabetes was 9%–25% when HbA1c was ≥5.7%, and this risk increased to 25%–50% for HbA1c ≥6%–6.5% [13]. This data formed the ADA’s basis in using HbA1c as screening criteria for T2DM/prediabetes in 2010. Since the acceptance of HbA1c as a screening tool, there has been a tremendous appeal among pediatricians to evaluate HbA1cs.
1. Benefits and pitfalls of HbA1c
HbA1c is not a direct measure of glycemia. It is an indirect measure, and it represents the amount of hemoglobin with glucose attached to the N-terminal valine of the beta chain, thus reflecting blood glucose levels over the previous 2–3 months (based on red blood cell turnover). HbA1c must be performed using a method that is certified by the National Glycohemoglobin Standardization Program (www.ngsp.org). The major advantages for HbA1c are that it does not require fasting, it has less pre-analytical and glycemic variability, and it is not affected by stress or illness. However, HbA1c has lower sensitivity and a higher cost, and it is availability is limited in many developing countries.
2. Nonglycemic factors that affect HbA1c levels in youth
(1) Red blood cell (RBC) turnover: Lower HbA1c values are observed in conditions of high RBC turnover, such as cystic fibrosis [14], hemorrhage, and hemolysis, and higher HbA1c values are seen when RBC turnover is slowed down, as in spherocytosis and iron deficiency anemia [15].
(2) Medications: Retroviral drugs [16], dapsone, hydroxyurea, and vitamins C & E can falsely lower HbA1c, and aspirin can interfere with the laboratory assay and artificially raise the HbA1c value [16].
3. Hemoglobinopathies
Hemoglobin variants, such S, C, D, and E, are substitutions for the B chain and can interfere with certain assays. More information about hemoglobinopathies is available at ngsp.org. African American youth who are heterozygous for the S trait could have an average HbA1c which is 0.3% higher for any given glucose level [17]. When corrected for age, body mass index (BMI), sex, and blood pressure, the HbA1c levels in African Americans is higher compared to Caucasians, which could be the result of inherent differences in glycation triggers besides the racial differences in the presence of traits [17].
4. Age
There is an age-related linear increase in HbA1c values for the same levels of glycemia, suggesting more efficient glycation in youth compared to adults [18].
Is HbA1c the ideal test?
After HbA1c came to the forefront as a screening test in 2010, several pediatric studies reported the validity of the HbA1c.
Nowicka et al. [19] compared HbA1c to the gold standard OGTT in over 1,000 patients. Of the 347 obese youth with prediabetes on the OGTT, 240 subjects had an HbA1c below the cutoff value of 5.7%. This study highlighted the unacceptably low sensitivity of HbA1c as a screening test. Brar et al. [6] studied 149 obese youth who were referred for HbA1c values ≥ 5.7% who then underwent an OGTT. At a sensitivity of 75% and a specificity of 57%, HbA1c has been unreliable due to its high false positive rate.
Buse et al. [20] studied 3,980 sixth-grade youth and found that HbA1c and IFG as screening tools defined different ethnic/racial groups. Elevated HbA1c was associated with the Hispanic and Black races, strong family history of diabetes, higher BMIs, higher waist circumferences and higher insulin values. High risk HbA1c was seen in 128 subjects (3.2%), and abnormal FPG was observed in 635 subjects (16%). IFG was found in youth who were mostly Hispanic, it being fivefold more common when compared to HbA1c in these children. The authors, among others, proposed that these contradictions call into question the utility of HbA1c as a standalone screening test. The conversion rate to diabetes was 0.8% for HbA1c and 1.1% for FPG in a 2-year follow-up evaluation.
Several studies indicate that using adult cutoff points for HbA1c to predict T2DM/prediabetes significantly underestimates the prevalence of these conditions in the pediatric and adolescent population, and consequently, a lower HbA1c cutoff point has to be proposed for children [19,21].
How do these tests do when compared to each other?
A retrospective review of 72 papers analyzed the comparisons of these three tests in youth. Table 3 highlights the findings of 5 papers.
Using FPG and HbA1c, Yang et al. [22] studied the prevalence of T2DM and/or prediabetes in 7,519 Chinese youth in grades 1, 7 and 10 in Shenzhen province. The cross-sectional study found a T2DM prevalence rate of 0.53% (n=4). Additionally, the prevalence of an abnormal FPG prevalence was 7 fold higher than the prevalence of an abnormal HbA1c (8.72% vs. 1.49%), and the odds ratio for an abnormal result for either test was higher for boys than girls (odds ratio, 1.21). As the authors did the testing on all youth irrespective of BMI, they found an interesting U-shaped curve, indicating higher rates of T2DM/prediabetes for underweight and obese youth. The proportion of prediabetes was higher for males than females, and the proportion decreased with grade for males but increased for females.
Al Amiri et al. [23] studied youth from 16 schools across the United Arab Emirates, a country with one of the highest prevalences of diabetes in the world. Of the 1,434 youth who were screened, 433 were overweight or obese. Among these 1,034 youth, 0.87% had T2DM. A significantly higher proportion of children had prediabetes using the results from the HbA1c criteria rather than the OGTT criteria (21.9 % vs. 5.4%). HbA1c results were discrepant for prediabetes but not for T2DM.
Nam et al. [24] simultaneously performed OGTT and HbA1c in 384 high risk overweight and obese youth across 6 hospitals in South Korea. The overall prevalence of T2DM and prediabetes was 18% and 31.1%, respectively. The authors proposed that HbA1c of 6.2% had a sensitivity of 91.5% and specificity of 93.7% with an area under the curve (AUC) of 0.972. For prediabetes 5.8 had a statistical significance with an AUC=0.795 (95% confidence interval [CI], 0.750–0.840), with a sensitivity of 64.1% and a specificity of 83.8% to screen for prediabetes. The authors found that 49 of the youths with T2DM fulfilled all three diagnostic criteria, and five youth fulfilled the OGTT criteria for T2DM but no the ADA’s HbA1c criteria of 6.5%. The authors demonstrated that the kappa coefficient of agreement between the two tests could be interpreted as fair (0.21–0.40) to moderate (0.41–0.60). For prediabetes, 9.4% of the youth fulfilled all the criteria, and 16.1% of the subjects would have missed without an OGTT as they only had an abnormal 2-hour PG. Because the pediatric cut-off value for HbA1c across racial/ethnic groups remains obscure, the authors recommended the combination of fasting and 2-hour glucose levels, in addition to HbA1c, as the best modality to screen for T2DM and/or prediabetes at this time.
Khokhar et al. [25] studied predominantly African American and Caribbean youth (n=230) and found that HbA1c had a receiver operating curve of 0.64 (95% CI, 0.56–0.72), showing that HbA1c had poor sensitivity for detecting prediabetes when compared to OGTT. While 56% of the cohort had an elevated HbA1c, 26% had an abnormal OGTT result, with 18% testing positive for both HbA1c and OGTT. All tests were done within a 3-month period. The authors proposed that HbA1c when combined with BMI z-score and homeostatic model of insulin resistance in a regression model was a better predictor of prediabetes in youth.
Ehehalt et al. [26] studied a large cohort of German youth (n=4,848) from several hospitals across Germany. The prevalence of T2DM was 0.02% (n=115), and these youth met one of the ADA criteria for T2DM. For those with T2DM, the OGTT had a sensitivity of 44.0% (95% CI, 30.0–58.7) and a specificity of 99.6% (95% CI, 99.3–99.7), whereas HbA1c had a sensitivity of 84.0% (95% CI, 70.9–92.8) and a specificity of 99.3% (95% CI, 99.0–99.5). The results were discordant for those whose 2-hour glucose levels were ≥200 mg/dL diabetes, median FPG, and median HbA1c levels were 110 mg/dL and 6.3 % (prediabetes) range. There was a high correlation (r=0.73) between FPG and 2-hour PG in those with confirmed T2DM (n=50) on repeat testing with either an OGTT and/or HbA1c. Prediabetes was highest with HbA1c criteria at 23% vs. FPG 12% vs. 2-hour PG 8%. In those youth with an abnormal HbA1c, the correlation between HbA1c and FPG was weak at r=0.18. However, the authors compared FPG+2-hour-PG (OGTT) vs. glycated hemoglobin and found an improvement in correlation with HbA1c (%) (log-transformed data, r=0.21, n=4,848, P<0.001). The authors surmise that these weak correlations were much lower than those reported in adults, possibly due to the fact that the tests measure different disorders of glucose metabolism. Moreover, the reproducibility of OGTT continues to be an area of controversy [26].
What are the inadequacies of the current diagnostic criteria for T2DM and/or prediabetes?
(1) The screening tests results are discrepant according to the ethnicity/race of the youth, so the "one size fits all" examination cannot be used for youth from different parts of the world.
(2) HbA1c as a standalone screening test for T2DM and/or prediabetes can result in the overdiagnosis of prediabetes, and many youths will have to undergo unnecessary OGTTs. The relevant literature contradicts the call for lowering HbA1c cut-off values for youth, as suggested by several studies. Youths with a high HbA1c above 6.2% must have an OGTT to establish the diagnosis of T2DM [27].
(3) Patients with a significant elevation of HbA1c ≥ 5.8%–6% or higher and normal OGTT results are at a higher risk for developing diabetes. The poor reproducibility of the OGTT in these situations may warrant testing HbA1c levels at three-month intervals, especially if the patient’s BMI is increasing.
(4) Although OGTT is not an "ideal test," it is the only test clinicians can use to assess prandial hyperglycemia. Youths with near-normal HbA1c values who have IGT will be missed if only FPG is tested.
Are there any alternatives to the current screening tests?
(1) Short-term markers of glycemia, such as 1,5-anhydroglucitol, glycated albumin and fructosamine, reflect glycemia ranging from 48 hours to 4 weeks, and they are promising for diabetes more so than for prediabetes screening [28]. Chan et al. [29] studied these markers in 14.3-year-old high risk youth (n=56) who were on a continuous glucose monitor. These markers predicted the glycemic variability as reported by the continuous glucose monitoring data independent of HbA1c.
(2) A 1-hour PG value of 155 mg/dL (8.6 mmol/L) has been assessed for identifying prediabetes in adults [30]. Jagannathan et al. [31] studied 212 adults and found the k coefficient of agreement for one-hour PG was 2 fold higher than HbA1c for prediabetes. Abdul-Ghani found that the receiver operating characteristic (ROC) curve for 1-hour PG was much higher than the HbA1c ROC curve for prediabetes (r=0.84 vs. 0.73) [32]. Kasturi et al. [33] studied the reproducibility and predictive value of 1-hour PG compared to the standard OGTT. Nondiabetic adolescent girls with obesity underwent a multiple-sample OGTT at baseline (n=93), 6 weeks (n=83), and 1 year (n=72). The shorter 1-hour OGTT provided the diagnostic equivalent of the standard OGTT with the advantage of being a shorter risk assessment.
Conclusions
HbA1c, FPG, and 2-hour PG provide a dynamic overview of different aspects of glucose homeostasis, although the results from these tests are often discrepant based on the race/ethnicity of the youth in which they are performed. Of note, when done together they can perform below their diagnostic threshold for prediabetes in 25% of youth for up to 2 years preceding the diagnosis of diabetes.
Risk-based screening for T2DM and/or prediabetes should be considered in youth at the onset of puberty or ≥10 years of age in overweight and obese youth who have 2 additional risk factors (Table 2). In high risk ethnic groups, such as African Americans and Hispanic youth, a FPG and/or OGTT may be more suitable than HbA1c. Based on our current literature review to date, there is limited data to support using HbA1c as a standalone screening test for high risk youth. As the pediatric cut-off values for HbA1c across racial/ethnic groups remain unclear, it is appropriate to recommend the combination of fasting and 2-hour glucose levels, in addition to HbA1c, as the best way to screen for T2DM and/or prediabetes at this time.
Notes
Conflict of interest No potential conflict of interest relevant to this article was reported.