Early-life exposure to endocrine-disrupting chemicals and pubertal development in girls
Article information
Abstract
Over the last decades, the onset of puberty in girls has occurred earlier, but the tempo of pubertal progression has been relatively slower, resulting in a younger age at puberty onset without a change in age at menarche. Sufficient energy availability and adiposity contribute to early pubertal development, and environmental factors, such as endocrine-disrupting chemicals (EDCs), may affect not only the control of energy balance, but also puberty and reproduction. EDCs are hormonally active substances that can perturb puberty by acting both peripherally on target organs, such as adipose tissue or adrenal glands, and/or centrally on the hypothalamic-pituitary-gonadal (HPG) axis. Depending on whether the exposure takes place earlier during fetal and neonatal life or later during early childhood, EDCs can lead to different outcomes through different mechanisms. Evidence of associations between exposures to EDCs and altered pubertal timing makes it reasonable to support their relationship. However, human epidemiologic data are limited or inconsistent and cannot provide sufficient evidence for a causal relationship between EDC exposure and changes in pubertal timing. Further investigation is warranted to determine the overall or different effects of EDCs exposure during prenatal or childhood windows on pubertal milestones and to reveal the underlying mechanisms, including epigenetic marks, whereby early-life exposure to EDCs affect the HPG-peripheral tissue axis.
Introduction
Puberty begins with the release of the hypothalamic gonadotropin-releasing hormone (GnRH) pulse generator from central nervous system inhibition after a quiescent period during childhood [1]. The age of menarche has definitely decreased from 16 years in the 1800s to 13 years in the 1960s, after which this downward trend seems to have slowed or even stopped [2]. The trend for earlier age at menarche has also been reported in Korea, from 16.6 years for females born between 1925 and 1929 to 12.6 years for females born between 1990 and 1994 [3,4]. While the onset of puberty has been occurring earlier, the overall tempo of pubertal progression is reported to be relatively slower, resulting in skewing towards younger ages for the onset of puberty and towards older ages for the completion of puberty [2]. Such increased duration of the pubertal transition may result from gonadotropin-independent estrogenic action at the level of the breast which may originate from peripheral tissues [5].
Although genetic factors remain the major determinant of pubertal timing [6], the secular trend for earlier onset of puberty has coincided with improved public health and nutrition [7-9]. Sufficient energy availability and early adiposity rebound contribute to early pubertal development. Endocrine-disrupting chemicals (EDCs) may affect not only the control of energy balance and adiposity, but also puberty and reproduction [2]. EDCs can act at any level in the 'hypothalamic-pituitary-gonadal (HPG)-peripheral tissues' axis [10]. Their effects can manifest right before puberty as well as much earlier, during fetal and neonatal life [2]. We reviewed the genetic and environmental factors that have been implicated in pubertal timing. We present the current evidence for the roles of EDCs on pubertal timing in girls based on human epidemiologic data and introduce potential mechanisms depending on the timing of exposure.
Factors implicated in pubertal timing by affecting the hypothalamic-pituitary-gonadal-peripheral tissues axis
Pubertal onset appears to be a highly heritable trait, but earlier sexual maturation in girls may be linked to environmental factors. Some genes implicated in precocious puberty (DLK1, MKRN3, and KISS1) and in delayed puberty (TACR3) are associated with variations in pubertal onset. The childhood GnRH pulse generator may be inhibited, in part, by MKRN3. Disinhibition of the HPG axis leads to the progressive amplification of pulsatile GnRH secretion which may be mediated by leptin levels and increased expression of neuropeptides, such as neurokinin B and kisspeptin, and their receptors [1]. In addition to central signals, peripheral metabolic and nutritional signals, such as leptin and insulin, play a permissive role in the proper function of the HPG axis [11].
Genes involved in pubertal timing or menarche reported by Elks et al. [12] and Perry et al. [13] include those related to hypothalamic GnRH secretion, pituitary development and function, hormone synthesis and bioactivity, energy homeostasis and growth, and potential peripheral feedback from sex steroids [13]. The overlap of genes involved in pubertal onset and obesity from genome wide association studies and human epidemiological data suggests a common pathway connecting early infancy weight gain, earlier pubertal onset, and later obesity in adulthood, although mechanisms whereby early-life changes in adiposity affect growth acceleration and early pubertal onset remain to be determined [14,15].
Obese girls are at risk for early pubertal development. Girls maturing at earlier ages have higher body mass index (BMI) z-scores with advanced Tanner stages [16]. Obesity-induced hyperinsulinemia and hyperleptinemia can affect linear growth and advance puberty [17]. Since adrenarche starts earlier as childhood BMI and insulin levels gradually increase, the increased adrenal androgen levels in obese children may also be responsible for accelerated growth before puberty [17]. In addition, hyperinsulinemia can increase sex steroid bioavailability via stimulation of ovarian growth and steroidogenesis, reduction of sex hormone binding globulin, and increased conversion of androgens to estrogens by stimulating aromatase activity in adipose tissues [2]. Breast development can occur from the gonadotropin-independent release of estrogen by peripheral tissues, such as the adrenal glands and adipose tissue. Finally, increased sex steroid levels in obese girls can induce gonadotropin-independent or dependent precocious puberty.
Gene-to-environment interactions during critical periods of development could alter gene expression in the HPG-peripheral tissue axis through epigenetic mechanisms [18]. While recent data have started to reveal the epigenetic regulation of KISS1 expression [18,19], epigenetic mechanisms that affect the HPG-peripheral tissue axis remain to be discovered. The intrauterine environment, birth size, nutrition, and (potentially) EDCs can affect adiposity and the timing of puberty. EDCs and adiposity may be the most concerning contributors to changes in puberty and reproduction. While sufficient energy availability provides clues to the mechanism of early pubertal development, changes in the control of both energy balance and reproduction may vary under the influence of common determinants, such as EDCs. These effects can manifest right before puberty as well as much earlier during fetal and neonatal life [20,21]. Although the link between EDCs and pubertal timing does not prove causality, their association is highly suggestive [22].
Possible mechanisms for the effect of EDCs on pubertal timing in girls
EDCs are defined as "an exogenous chemical, or mixture of chemicals, that interferes with any aspect of hormone action" by the Endocrine Society’s second scientific statement published in 2015 [10]. Endocrine disruptors can be naturally occurring or synthetic. This section reviews the effects of synthetic EDCs on pubertal timing in girls.
EDCs can act through several mechanisms at any level in the 'HPG-peripheral tissues' endocrine axis. Directly, EDCs may affect genes or HPG pathways unique to puberty. Indirectly, EDCs can act as obesogens and change metabolic programming during fetal and early childhood development, resulting in alterations in the metabolic and peripheral hormones associated with the onset of puberty [15]. A variety of chemical toxins that are ubiquitously present in our environment exert antagonistic and agonistic actions on hormonal axes and pathways at low versus high concentrations, and they demonstrate nonmonotonic dose-response curves [10]. Since many EDCs can act as agonists of estrogen receptors (ERs) or antagonists of androgen receptors (ARs), EDCs may mimic naturally occurring estrogens and androgens. In addition, EDCs might bind to a receptor within a cell and block the functions of endogenous hormones, thus acting as antiestrogens and antiandrogens [10,23].
Low-level exposures to a mixture of EDCs may mask the effect of an individual compound. Moreover, interindividual and interspecies variation in susceptibility may be present due to differences in pharmacokinetics and/or genetic polymorphisms in key genes, complicating our ability to translate data from animal studies to human health [20]. In humans, the long interval from exposure to endpoints (latent effects) and the setting of mixed exposures over a lifetime make it difficult to analyze the health outcomes of EDCs [20]. Furthermore, it is difficult to distinguish the influence of nutrition and adiposity on pubertal outcomes from that of EDCs. Since the prenatal, neonatal, infancy, early childhood and peripubertal periods are critical windows of development, EDCs may have different effects depending on the time of exposure [2]. Though the mechanisms whereby fetal or neonatal life changes pubertal timing have not been fully elucidated, EDCs can alter the genes involved in the modification of epigenetic marks [2,15]. Although researching the effect of EDCs on pubertal timing is difficult for the various aforementioned reasons, further investigation is warranted to determine which EDCs can affect pubertal timing, which periods of exposure are critical, and how different mechanisms are involved depending on the timing of exposure in humans [24].
Human epidemiologic data on EDCs in regards to pubertal timing in girls
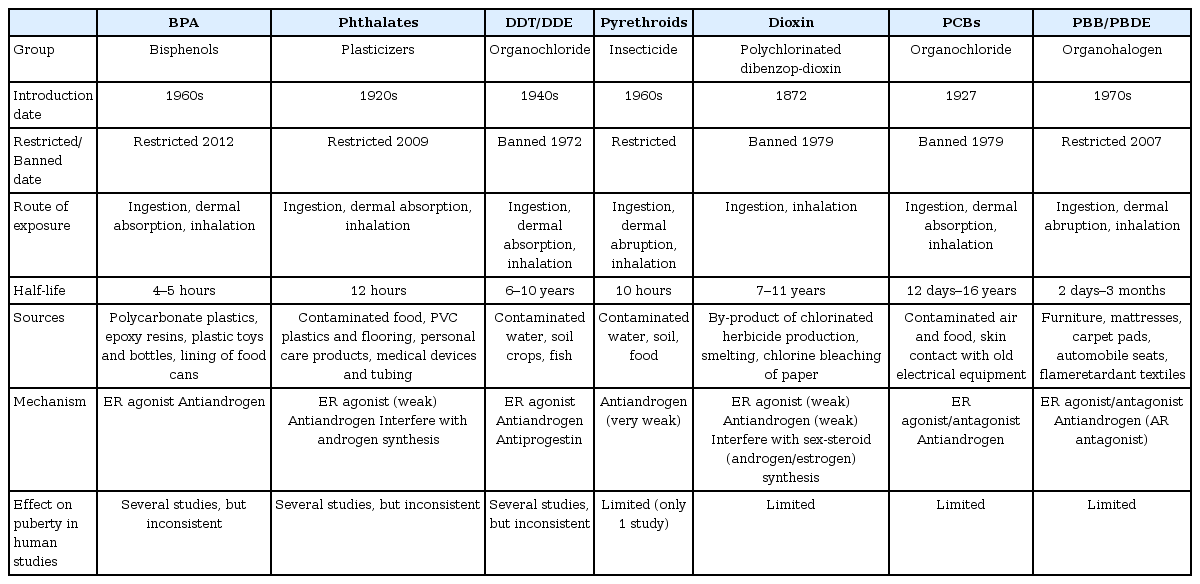
The Endocrine Society's Scientific Statements present a review of the literature on human studies that assessed the associations between EDCs exposure and pubertal timing [10]. There have been extensive reviews about the contribution of EDCs on shifts in pubertal timing [2,10,24,25] as well as a recent review of publications from 2016 to 2017 that examined EDC associations with pubertal milestones [26]. The EDCs most extensively studied in regards to puberty include bisphenol A (BPA), phthalate esters, pesticides (dichlorodiphenyl trichloroethane [DDT], pyrethroids), dioxins and polychlorinated biphenyls (PCBs), and flame retardants (polybrominated biphenyls [PBB], polybrominated diphenyl ethers [PBDEs]) (Table 1). This section reviews the human epidemiologic studies regarding the relationship of individual EDCs with pubertal development or menarche in girls.
Bisphenol A
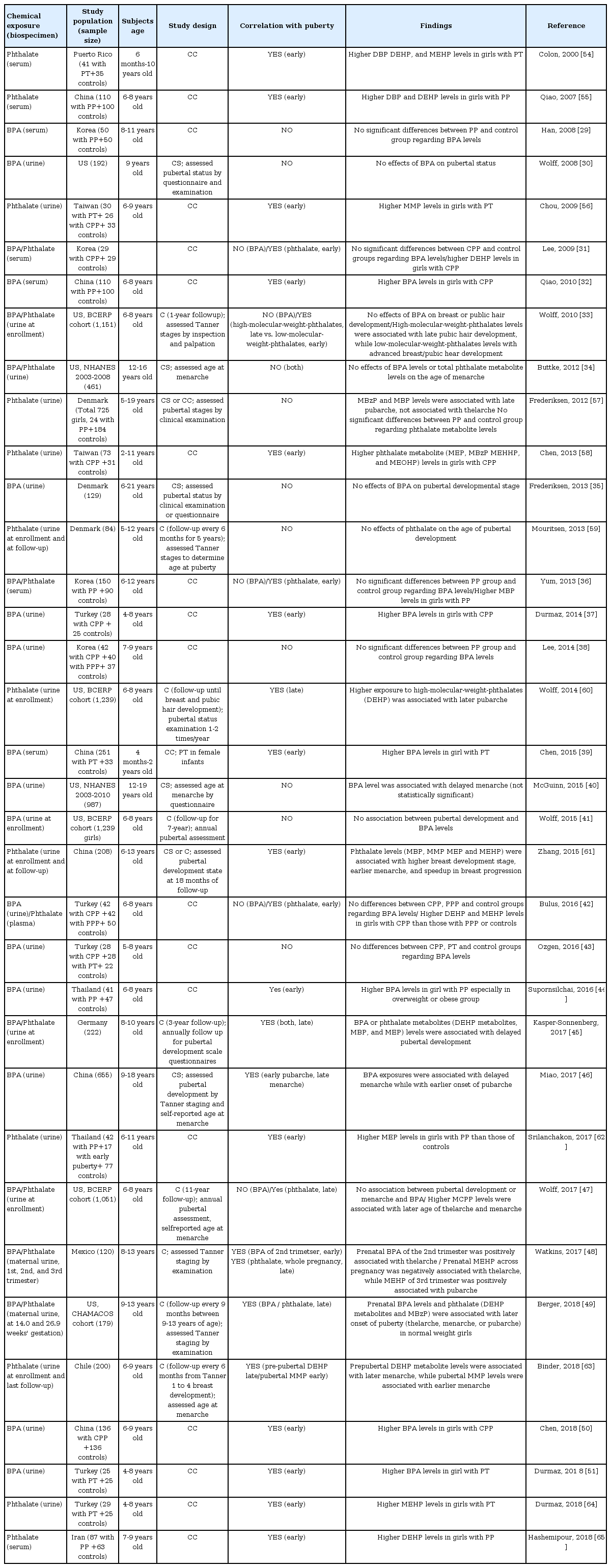
BPA is used in polycarbonate plastics, plastic bottles and toys, epoxy resins, dental sealants, and linings of food cans and has a half-life of 4 to 5 hours [27]. BPA has weak ER agonist activity at lower concentrations but can also compete with endogenous estrogens for binding, and they have antiandrogenic properties at higher concentrations [28]. Epidemiological studies on BPA and puberty have produced inconsistent results (Table 2) [29-51]. Serum or urinary BPA concentrations were significantly associated with premature thelarche [39,51], precocious puberty [32,37,44,50], and earlier pubertal development [48]. However, other studies have reported no significant relationship [29-31,33-36,38,40-43,47] or later onset of puberty or menarche [45,46,49]. Although most previous studies for BPA and pubertal milestones were limited by cross-sectional or case-control study designs, 6 longitudinal studies have been reported [33,41,45,47-49]. Early childhood exposure to BPA showed no relationship to pubertal timing [33,41,47] or late onset of puberty [45]. Prenatal exposure was associated with earlier thelarche [48], but rather later thelarche, pubarche, and menarche [49]. Recently in 2017, a critical review on the effect of BPA on puberty [52] concluded that the current available data cannot establish a clear role for BPA in pubertal development due to conflicting results among the clinical and epidemiological studies examined.
Phthalates
Phthalates are used as liquid plasticizers in plastics and flooring, personal care products, medical devices and tubing with half-lives of 12 hours. Although the mechanism remains to be further elucidated, phthalates might act as ER agonists or AR antagonists and may also interfere with androgen synthesis [53]. Phthalates may be classified into low-molecular-weight phthalates (ester side-chain lengths, 1 to 4 carbons) and high-molecular-weight-phthalates (ester side-chain lengths, 5 or more carbons). Di(2-ethylhexyl) phthalate (DEHP) metabolites (mono(2-ethyl-5-oxohexyl) phthalate and mono(2-ethyl-5-hydroxyhexyl) phthalate), which are classified into high-molecular-weight-metabolites, were the phthalates most commonly studied.
The results from studies regarding the relationship between phthalate and puberty in girls conflict depending on the timing of exposure and/or different phthalate metabolites (Table 2) [31,33,34,36,42,45,47-49,54-65]. Different studies have shown significant associations with premature thelarche [54,56] and precocious or early puberty [31,36,42,55,58,61,62,64,65], while no relationship with puberty [34,57,59] or an association with later onset of puberty [45,47-49,60] have also been reported. Among several studies on phthalate exposure, nine studies had a longitudinal cohort design [33,45,47-49,59-61,63]. Prenatal exposure to high-molecular-weight phthalate metabolites was associated with later onset of puberty [48]. Early childhood exposure to phthalate metabolites was not associated to puberty [59] or associated with earlier [61] or later [45,47,49,60] pubertal progression. Meanwhile, differences in pubertal development or age at menarche has been reported depending on the timing of exposure or classes of exposures (high or low-molecular-weight-phthalates) [33,63]. Whereas high-molecular-weight phthalate levels were associated with later pubic hair development, low-molecular-weight phthalate levels were related to advanced breast or pubic hair development [33]. While prepubertal exposure to DEHP was associated with later menarche, pubertal exposure to monomethyl phthalate (low-molecular-weight) was related to earlier menarche only among overweight or obese girls and not among those that were of normal weight [63].
Pesticides
Pesticides are substances used to kill or reduce the quantity of insects, weeds, rodents, or fungi. They are classified as insecticides, herbicides, fungicides, and rodenticides depending on their target. While over 100 pesticides are identified as EDCs, DDT and its metabolite dichlorodiphenyl dichloroethane (DDE) are the most extensively studied compounds [66]. DDT/DDE have estrogenic, antiandrogenic, and antiprogestin effects [67]. DDT is a persistent organic pollutant with a half-life of 6–10 years. Although many, but not all, countries have banned organochlorine pesticides, it is insoluble in water and very persistent in the environment [68,69]. The results of human studies on the relationship between DDT exposure and puberty are inconsistent (Table 3) [30,70-78]; results have ranged from no relation to puberty [30,72,73,75,78] to associations with precocious puberty [74], earlier age at menarche [70,71], and later onset of puberty [76,77]. Three longitudinal studies have revealed that higher exposure to DDE prenatally was associated with early onset of puberty [70] or no relation to puberty [78], whereas higher exposure in childhood was related to later onset of puberty [76].

Human studies regarding the relationship of pyrethroids, DDT/DDE, dioxins, PCBs, and flame retardants with pubertal development in girls
Pyrethroids are the most commonly used residential insecticides, having replaced some of the agricultural use of organophosphorus and carbamate insecticides. Pyrethroids are quickly metabolized and eliminated through the kidneys in humans, and their half-life is less than 10 hours [79]. The most frequently utilized pyrethroid biomarker is 3-phenoxyboenzoic acid, and it has been reported as weakly androgenic [80]. One cross-sectional study [81] reported an association between pyrethroids exposure and later onset of puberty in girls.
Dioxins and PCBs
Dioxins are byproducts of manufacturing processes, including smelting, bleaching of paper pulp, and the manufacturing of herbicides and pesticides. The chemical name for dioxin is 2,3,7,8-tetrachlorodibenzo para dioxin (TCDD). The name "Dioxins" is often used in chemically and structurally similar compounds, such as polychlorinated dibenzodioxins (PCDDs) and polychlorinated dibenzo furans. Dioxins are known to have estrogenic and antiandrogenic activities, and their half-life is 7–11 years in the body [82]. In addition, PCBs also constitute a group of these polychlorinated aromatic hydrocarbons and have similar toxic properties with dioxin. PCBs have estrogenic and antiandrogenic effects [83], and the maximum elimination half-life for PCBs is approximately 10–15 years [84]. The few studies on the relevance of dioxins/PCBs to puberty (Table 3) [70,72,73,76,85-87] have shown inconsistent and limited data. They have shown a positive association with early onset of menarche [72] as well as with delayed pubertal development [73,85]. Two longitudinal cohort studies have reported different outcomes: one study reported no association between prenatal PCBs exposure and menarcheal age [70], while the other reported a significant correlation between prenatal PCDD/Fs exposure and delayed breast development [87]. Other prospective studies on childhood TCDD exposure have reported no associations with pubertal development [76] or the onset of menarche [86].
Polybrominated flame retardants
Flame retardants refer to a variety of substances that are added to materials to prevent the start and growth of fires. With approximate half-lives of 2–7 days, they are used in many products, such as furniture, mattresses, carpets, and flame-retardant textiles [88]. Polybrominated flame retardants include compounds such as PBDEs, PBB, and so on [88]. Estrogenic and anti androgenic activities have been reported with exposure to these compounds [89]. To date, there have been limited studies on pubertal timing, with inconsistent results that depend on the timing of exposure (Table 3) [76,87,90-93]. The limited studies have shown positive associations with premature thelarche [92], earlier pubertal development [90], and age at menarche [91], although associations with later onset of puberty [76,93] or no association [87] have also been reported. Three prospective studies have reported contrasting outcomes in which prenatal PBB/PBDE exposures showed no association [87] or associations with both earlier [90] and later [93] pubertal timing. Another prospective study reported an association between childhood PBDE exposure and later onset of puberty [76].
Revised paradigm of EDCs on pubertal timing and future directions
Parent et al. [2] suggested a revised paradigm of EDCs on pubertal timing. Since improvements in public health and nutrition have paralleled a downward trend in pubertal timing and menarche, environmental factors are the predominant determinants in pubertal timing. Classically, environmental factors, including EDCs, might advance pubertal timing via central mechanisms, particularly during the prepubertal period. According to the revised paradigm [2], environmental factors can influence puberty and reproduction through not only central but also peripheral mechanisms, depending on whether they take place early during fetal and neonatal life or late during the prepubertal period. Environmental factors can affect energy availability during the fetal and neonatal period or in infancy and early childhood. They can also act as obesogens and promote early adiposity rebound, leading to changes in metabolic or peripheral signals and increases in adrenal androgen levels, with subsequent early pubertal development. Environmental factors, including EDCs, might affect genetic or epigenetic pathways during critical windows of development. Further investigation is warranted in order to reveal the underlying mechanisms whereby early-life exposure to EDCs affects the HPG-peripheral tissue axis.
Conclusion
The onset of puberty in girls is occurring earlier, without changes in the timing of completion of puberty. Physiological variability and multiple other factors affect the initiation of puberty. Exposure to a broad mixture of EDCs is ubiquitous, and the shifts in pubertal timing may be mediated by exposures to EDCs at critical developmental windows. EDCs are hormonally active substances that can act via several mechanisms to perturb puberty either peripherally on target organs (adipose tissue or adrenal glands) or centrally via the HPG axis. EDCs can influence pubertal timing through different mechanisms depending on when the exposure occurs during early-life. Evidence for associations between exposures of EDCs and altered pubertal timing makes it reasonable to support the hypothesis that a relationship between EDCs and pubertal timing exists. However, the current data is insufficient and conflicting to provide sufficient evidence for a causal relationship between EDCs exposure and changes in pubertal timing in humans. Further human epidemiologic studies of prospective and longitudinal design are needed to determine the combined effect of EDC exposure on puberty and reproduction during critical periods. In addition, the underlying mechanisms whereby early-life exposures to EDCs influence puberty, including epigenetic marks, need to be explored in further studies.
Notes
Conflict of interest No potential conflict of interest relevant to this article was reported.
Acknowledgements
This study was supported by grants from the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education (2018R1D1A1B07049806).