Low levels of 25-hydroxyvitamin D in children and adolescents with type 1 diabetes mellitus: a single center experience
Article information
Abstract
Purpose
Low vitamin D level is common in adults with diabetes mellitus (DM). We assessed vitamin D level and its associated factors in Korean youth with type 1 DM.
Methods
Type 1 DM cases (n=85) and healthy controls (n=518) aged <20 years were included and grouped into 3 categories according to vitamin D level: deficiency (<20 ng/mL), insufficiency (20–30 ng/mL), or sufficiency (≥30 ng/mL).
Results
The mean serum vitamin D level was significantly lower (21.6±8.5 ng/mL vs. 28.0±12.0 ng/mL, P<0.001) and vitamin D deficiency prevalence was significantly higher (48% vs. 26%, P<0.001) in type 1 DM cases than in healthy controls. Logistic regression analysis revealed that type 1 DM cases were more likely to have vitamin D deficiency (P=0.004), independent of sex, age, and body mass index. Type 1 DM cases with vitamin D deficiency/insufficiency were mainly diagnosed in winter (November to April) (P=0.005), and the duration of diabetes was longer than in those with vitamin D sufficiency (P=0.046). However, season of diagnosis, duration of diabetes, prescribed daily insulin dose, and glycosylated hemoglobin and C-peptide levels were not associated with 25-hydroxyvitamin D (25(OH)D) level in type 1 DM cases after adjustment for other factors.
Conclusions
We recommend assessment of serum 25(OH)D level in type 1 DM cases and to treatment if findings indicate insufficiency. Further studies investigating the mechanisms underlying vitamin D deficiency in youth with type 1 DM are needed.
Introduction
Type 1 diabetes mellitus (DM) is an autoimmune disease; it results from the destruction of insulin-secreting pancreatic beta cells [1]. Vitamin D is traditionally associated with calcium metabolism, and bone growth [2]. Recent studies have identified potential roles of vitamin D in several nonskeletal disorders, including, T-cell-mediated immune disease, DM, and cardiovascular diseases [3,4]. Vitamin D receptors are expressed in the pancreas and on activated T lymphocytes. Additionally, vitamin D is thought to modulate T-cell activation and its cytokines [3].
Previous epidemiologic and in vitro studies suggest the association between vitamin D deficiency and an increased incidence of type 1 DM. The incidence rates of type 1 DM in children are associated with latitude gradient, which is inversely associated with ultraviolet B irradiation amount [5]. The increased incidence of type 1 DM coincides with a decrease in the recommended vitamin D intake according to a Finnish birth-cohort study [6]. Long-term administration of high dose vitamin D suppresses the incidence of DM in nonobese diabetic mice [7]. Several recent studies in Western countries, have shown that vitamin D levels are lower in type 1 DM youth [8,9]. Racial variations are seen in the etiology and incidence of type 1 DM in Western and Asian countries; however, few studies have determined the association between vitamin D level and type 1 DM in Northeast Asian children and adolescents.
Therefore, our aim was to assess the vitamin D levels in Korean youth with type 1 DM and compare these levels with those in healthy controls. Additionally, we evaluated the associations between vitamin D levels and other parameters in type 1 DM cases.
Materials and methods
1. Subjects
For this study, 603 children and adolescents aged between 6 and 20 years, who visited the Department of Pediatrics at Korea University Hospital in 2011 were recruited. The subjects included 85 patients with type 1 DM, and 518 healthy controls. Participants with a history of vitamin D supplement or drug use that could affect serum vitamin D levels, such as anticonvulsants or systemic glucocorticoids, and those with a past history of chronic kidney or hepatic diseases were excluded.
2. Methods
The sex, age, height, body weight, body mass index (BMI, kg/m2), and laboratory profiles of the subjects were recorded. BMI was expressed as a standard deviation score (SDS) for age-and sex- matched Korean youth according to the 2007 Korean national growth chart. Detailed history of medication drug intake, systemic diseases, and average daily duration of outdoor activity were recorded. Additionally, duration of diabetes, prescribed daily insulin dose (IU/kg), season of diagnosis, and glycosylated hemoglobin (HbA1c) and C-peptide levels in youth with type 1 DM were recorded. The seasons of type 1 DM diagnosis were classified as follows: summer (May to October) and winter (November to April).
Serum calcium, phosphorus, magnesium, alkaline phosphatase, and parathyroid hormone levels were also measured. Blood was drawn and always kept in the dark during processing. Plasma was immediately separated and analyzed. Serum 25-hydroxyvitamin D (25(OH)D) levels were measured using a radioimmunoassay kit (DIAsource, Nivelles, Belgium) and reported as ng/mL. Serum 1α, 25-dihydroxyvitamin D levels were measured using a radioimmunoassay kit (DiaSorin, Stillwater, MN, USA) and reported as pg/mL. Since vitamin D levels are associated with sunlight exposure, which varies by season, blood samples of youth with type 1 DM were collected from June to August. We categorized 25(OH)D status as deficiency (<20 ng/mL), insufficiency (≥20–30 ng/mL), or sufficiency (≥30 ng/mL) based on the Endocrine Society Clinical Practice guideline [10]. None of the subjects had a history of bone deformity or fracture.
3. Statistical analysis
IBM SPSS Statistics ver. 20.0 (IBM Co., Armonk, NY, USA) was used. Independent t-test and Mann-Whitney U-tests were used to compare the 2 groups. One-way analysis of variance (ANOVA) and repeated measures ANOVA were conducted to compare the clinical and biochemical parameters of the vitamin D subgroups. Chi-square test was used to assess vitamin D deficiency prevalence. The odds ratio (OR) and 95% confidence interval (CI) for the risk of having type 1 DM were calculated using logistic regression analysis, after adjustment for potential confounding variables. Uni- and multivariate linear regression analyses were performed to evaluate the association between the 25(OH)D levels and other variables. All data were expressed as mean±standard deviation, and P<0.05 was considered statistically significant.
4. Ethics statement
This study was reviewed and approved by the Institutional Review Board of the Korea University Anam Hospital (approval number: AN11182). Written informed consent was obtained from the subjects and their parents.
Results
1. Clinical and biochemical characteristics of youth with type 1 DM and healthy controls
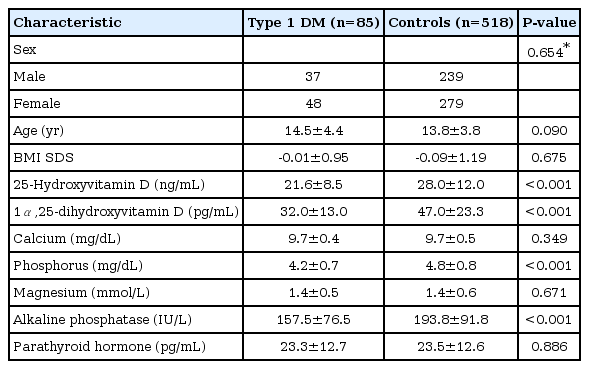
Data were obtained for 85 youth with type 1 DM (37 boys, 48 girls) and 518 healthy controls (239 boys, 279 girls). The mean ages of type 1 DM cases and controls were 14.5±4.4 years and 13.8±3.8 years, respectively. Serum 25(OH)D levels were significantly lower in the type 1 DM cases than in healthy controls (21.6±8.5 ng/mL vs. 28.0±12.0 ng/mL, P<0.001). Alkaline phosphatase, phosphorus, and 1α, 25-dihydroxyvitamin D levels were also significantly lower in the type 1 DM cases (P<0.001 as compared with healthy controls). The age, sex, BMI SDS, calcium, magnesium and parathyroid hormone levels were similar in the 2 groups (Table 1).
2. Prevalence of vitamin D deficiency in youth with type 1 DM and healthy controls
Of the 603 subjects, 177 (29%) had vitamin D deficiency, 236 (39%) had vitamin D insufficiency, and 190 (32%) had vitamin D sufficiency (Table 2). Vitamin D deficiency prevalence was considerably higher in type 1 DM cases than in healthy controls (48% vs. 26%, P<0.001).
3. Logistic regression analysis of the association between having type 1 DM and the vitamin D categories
Logistic regression analysis of the increased risk of having type 1 DM according to the vitamin D categories is shown in Table 3. Children and adolescents with type 1 DM were more likely to have vitamin D deficiency (OR, 3.79; 95% CI, 1.99–7.24; P<0.001). After adjustments for age, sex, and BMI SDS, vitamin D deficiency remained significantly associated with having type 1 DM (OR, 3.72; 95% CI, 1.52–9.10, P=0.004). The risk of having type 1 DM increased further after adjustments for age, sex, BMI SDS, alkaline phosphatase, calcium, magnesium, phosphorus, and parathyroid hormone levels in vitamin D deficiency (OR, 6.06; 95% CI, 1.28-28.66; P=0.023). However, vitamin D insufficiency was not associated with presence of type 1 DM.
4. Clinical and biochemical characteristics of youth with type 1 DM according to the vitamin D categories
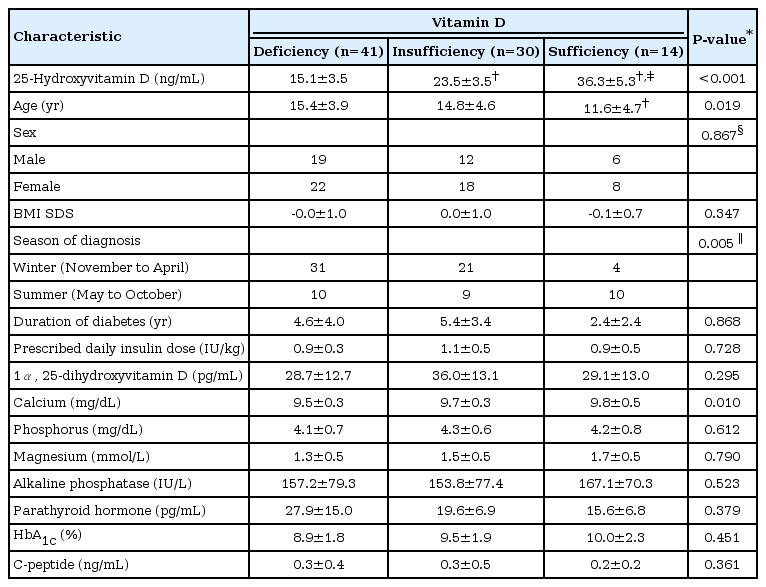
The mean age of the type 1 DM cases was lowest for those with vitamin D sufficiency, among the 3 categories (15.4±3.9 for those with vitamin D deficiency vs. 14.8±4.6 for those with vitamin D insufficiency vs. 11.6±4.7 years for those with vitamin D sufficiency, P=0.019) (Table 4). The sex ratios and BMI SDS were similar for the 3 categories. At initial type 1 DM diagnosis, 76% of vitamin D deficiency cases were diagnosed in winter, in contrast with only 29% of vitamin D sufficiency cases (P=0.005). The mean duration of diabetes and prescribed daily insulin dose were 4.5±3.7 years and 1.0±0.4 IU/kg, respectively. The disease duration of type 1 DM in case of vitamin D sufficiency was shorter than that in case of vitamin D insufficiency (2.4±2.4 years vs. 5.4±3.4 years, P=0.046); however, there was no significant difference among the 3 categories after adjusting for age (P=0.868). The mean prescribed daily insulin dose was similar among the 3 categories.
The mean 25(OH)D levels in the deficiency, insufficiency, and sufficiency categories were 15.1±3.5, 23.5±3.5, and 36.3±5.3 ng/mL, respectively (P<0.001). The mean calcium level was significantly lower in the vitamin D deficiency category than in the other categories (P=0.010), while the mean parathyroid hormone level was similar among the 3 categories after adjusting for age (P=0.379). On initial diagnosis and recent analysis, the mean HbA1c levels were 12.4%±2.8% and 9.3%±1.9%, respectively, and the mean serum C-peptide levels were 0.5±0.5 ng/mL and 0.3±0.4 ng/mL, respectively. The mean HbA1c and C-peptide levels were similar among the 3 categories.
5. Linear regression analysis of factors associated with 25(OH)D levels in youth with type 1 DM
On univariate analysis, serum 25(OH)D levels were negatively correlated with age (β=-0.594, P=0.004), parathyroid hormone levels (β=-0.200, P=0.009), and duration of diabetes (β=-0.583, P=0.023). Serum 25(OH)D levels were positively correlated with calcium levels (β=11.221, P<0.001), magnesium levels (β=3.816, P=0.031), and diagnosis in summer (β=4.101, P=0.033). However, serum 25(OH)D levels were not associated with BMI SDS, prescribed daily insulin dose, and HbA1c and C-peptide levels. Results of multivariate linear regression analysis after adjustment for age, sex, BMI SDS, calcium, phosphorus, magnesium, alkaline phosphatase, and parathyroid hormone levels, are shown in Table 5. After adjustment, serum 25(OH)D levels showed no significant correlation with diagnosis in summer, duration of diabetes, prescribed daily insulin dose, and HbA1c and C-peptide levels.
Discussion
In this case-control study, we found that serum 25(OH)D levels of the type 1 DM cases were significantly lower and vitamin D deficiency prevalence was considerably higher than in healthy controls. Cases of type 1 DM with vitamin D deficiency/insufficiency were mainly diagnosed in winter, and the duration of diabetes was longer in these cases than in those with vitamin D sufficiency. However, there was no association among serum 25(OH)D levels, season of diagnosis, duration of diabetes, prescribed daily insulin dose, and HbA1c and C-peptide levels after adjusting for age, sex, BMI SDS, calcium, phosphorus, magnesium, alkaline phosphatase, and parathyroid hormone levels.
The nonskeletal effects of vitamin D on T-cell immunity have attracted attention in addition to its known skeletal role in rickets and osteomalacia [3,4]. Vitamin D plays an active role in regulation of the immune system via its receptors located in several tissues, including beta-cells and T lymphocytes [8].
In the present study, youth with type 1 DM had significantly lower serum 25(OH)D levels than healthy controls. Several confounding factors may have influenced these relationships. Factors related to serum 25(OH)D levels, including vitamin D intake and its seasonal variations, were excluded. Serum 25(OH)D level increased by 0.7 ng/mL for every additional daily intake of 100-IU cholecalciferol [11]. In this study, the difference in the mean 25(OH)D levels between the 2 groups was 6.4 ng/mL, equivalent to 914 IU of cholecalciferol daily. Thus, the possibility of inadequate vitamin D intake alone cannot explain the low 25(OH)D levels in youth with type 1 DM. As vitamin D3 is synthesized from 7-dehydrocholesterol in the skin on exposure to sunlight [12], serum 25(OH)D levels are usually higher in summer than in winter [13]. Although we collected samples from all the youth with type 1 DM in summer, blood sampling in healthy controls was not limited to the summer alone because of practical difficulty. Considering the seasonal variations in 25(OH)D levels, the difference in levels could be greater between the 2 groups. Johnson et al. [14] reported that youth with low vitamin D levels had higher fasting glucose levels. Previous cross-sectional studies have also shown lower 25(OH)D levels among newly diagnosed type 1 DM cases [15]; the mean vitamin D level was lower in American and Swedish youth with type 1 DM [16,17]. Prevalence of vitamin D deficiency was higher in 170 Qatari children with type 1 DM compared to nondiabetic children [18]. A meta-analysis of total 1,900 children and adolescents (mean age, 10.9 years) from 11 studies reported a 25(OH)D level that was 5.69 ng/mL lower than that in nondiabetic controls [19]. These meta-analyses did not include youth with type 1 DM from Northeast Asia [20,21]. There are very few studies including Northeast Asian youth with type 1 DM. Although previous studies included a small number of subjects and did not include factors such as season of diagnosis and duration of disease, they reported no significant differences in vitamin D levels among Korean youth with type 1 and 2 DM compared to nondiabetic controls [21].
In this study, vitamin D deficiency prevalence was significantly higher in youth with type 1 DM than in healthy controls. The risk of type 1 DM increased after adjustment for sex, age, BMI SDS, and other serum variables in vitamin D deficient subjects. Vitamin D insufficiency/deficiency prevalence were observed in 15% to 61% of youth with type 1 DM in Western countries [16]. On the other hand, no significant association was found between serum 25(OH)D levels and type 1 DM in some studies [22,23]. This difference can possibly be explained by the different sample size, as well as racial, and skin type variations.
Universal findings of low vitamin D levels in patients with type 1 DM may indicate a role in the pathogenesis of diabetes; however, mechanisms underlying vitamin D deficiency in patients with type 1 DM remain unclear. Several studies have also suggested an immuno-modulatory effect of vitamin D in both T-helper 1 and 2 diseases [24,25]. Both in vitro and animal studies have shown that vitamin D suppresses T-helper 1 responses and stimulates T-helper 2 responses [26]. The excretion of vitamin D-binding protein is increased in the urine of megalin-deficient mice, suggesting that synchronous urinary loss of vitamin D-binding protein and megalin ligand can lead to vitamin D deficiency [27,28].
In this study, serum 25(OH)D levels in type 1 DM cases were negatively correlated with age, and parathyroid hormone level, and positively correlated with calcium, and magnesium levels. Svoren et al. [16] also reported a negative correlation between 25(OH)D level and age in type 1 DM cases. Increased parathyroid hormone levels in vitamin D deficiency constitute a feedback mechanism secondary to decreased vitamin D levels [29]. Although certain studies reported low 25(OH)D levels in obesity [30], 25(OH)D levels were not related with BMI SDS in the present study since type 1 DM youth were not obese.
In this study, youth with vitamin D deficiency were diagnosed with type 1 DM in winter more frequently than those with vitamin D sufficiency. Italian youth with type 1 DM had lower vitamin D levels unassociated with seasons of birth and visit [31]. Seasonal variations such as the high incidence of type 1 DM in autumn and winter, were found in Danish children [32]. In contrast to previous studies, this study assessed the association between season of diagnosis and vitamin D deficiency in type 1 DM cases.
Janisse et al. [33] reported that children with insufficient vitamin D had poor glycemic control. Daily insulin requirements were higher in Turkish type 1 DM children with low 25(OH)D levels [34]. The precise mechanism of the association between 25(OH)D levels and the severity of type 1 DM has not been elucidated. Low 25(OH)D levels were found to be associated with impaired β-cell function as well as an increased risk of insulin resistance [35]. However, relationship between 25(OH)D levels, disease duration, mean prescribed daily insulin dose, and recent HbA1c and C-peptide levels were not exist among the 3 vitamin D categories in the type 1 DM cases after adjustment for other factors. Similarly, other studies also reported no relationship between 25(OH)D, HbA1c level, mean prescribed daily insulin dose, and duration of diabetes in type 1 DM patients [8,16,36].
This study had some limitations. First, data were limited because of the cross-sectional study design. Second, information about the amount of sunlight exposure and socioeconomic state was not available. The amount of sunlight exposure was assumed based on information on outdoor activities. Despite these limitations, this study had several strengths. The youth with type 1 DM in the present study lived along the same latitude, and blood sampling was conducted during the summer. The findings of this study may serve as basis for further supporting data on Korean youth with type 1 DM.
In summary, the mean serum 25(OH)D level was significantly lower and vitamin D deficiency prevalence was significantly higher in type 1 DM youth than in healthy controls. Cases of type 1 DM with vitamin D insufficiency/deficiency were mostly diagnosed in winter, and duration of diabetes was longer in these cases than in those with vitamin D sufficiency. There was no relationship between 25(OH)D levels and duration of diabetes, prescribed daily insulin dose, and HbA1c and C-peptide levels after adjustment for other factors. We recommend assessment of serum 25(OH)D levels in type 1 DM youth and prompt treatment if findings indicate insufficiency. The role of the pathogenesis of diabetes in the vitamin D statuses of patients with type 1 DM needs to be elucidated in further studies.
Notes
Conflict of interest: No potential conflict of interest relevant to this article was reported.