Predictive factors for early response to methimazole in children and adolescents with Graves disease: a single-institute study between 1993 and 2013
Article information
Abstract
Purpose
We aimed to investigate the predictive factors for early response to methimazole (MMI) in pediatric patients with Graves disease (GD).
Methods
Our study included 44 pediatric patients who were diagnosed with GD between January 1, 1993, and December 31, 2013, and were available for follow-up, achieving a normalization of thyroid functions (TFs) at the Chonbuk National University Hospital Pediatric Department. We retrospectively analyzed TFs such as tri-iodothyronine (T3), free thyroxine (fT4), thyroid-stimulating hormone (TSH), and thyroid antibody levels at diagnosis. We also examined their family history of thyroid disease, symptoms at presentation, and normalization time for TF after treatment. We divided our clinical series of patients into the following 4 age groups: <7 years old, 7–12 years old, 13–15 years old, and 16–18 years old.
Results
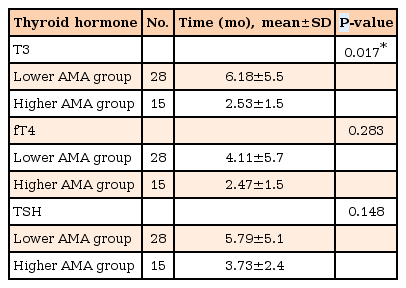
At diagnosis, the time of normalization of T3 was significantly shorter in the higher antimicrosomal antibody (AMA) group compared with the lower AMA group (2.53 months vs. 6.18 months) (P<0.05). However, the time of normalization of T3/fT4/TSH had no significant correlations with other variables such as age, sex, a family history of thyroid diseases, thyroglobulin, thyroid-stimulating immunoglobulin, or antithyroglobulin antibody (ATA).
Conclusion
Higher serological titers of AMA at diagnosis may have prognostic value in the response to initial MMI treatment in pediatric hyperthyroid GD patients.
Introduction
Thyroid diseases are the most common endocrine diseases in children; they are characterized by impaired synthesis or functions of thyroid hormones due to genetic aberrations during growth and development1). Thyroid dysfunction leads to impairment in growth and development during childhood and metabolic dysfunction in adults2).
Hyperthyroidism is characterized by the overproduction of thyroid hormone, and its incidence is lower in children compared with adults, i.e., pediatric thyroidism accounts for approximately 5% of all diagnosed cases345). In contrast, more than 95% of pediatric hyperthyroidism evolves from Graves disease (GD), also known as diffuse toxic goiter6). Currently, antithyroid medications are considered the first-line treatment for pediatric hyperthyroid GD78). However, it remains problematic because treatment occurs over a long period of time and high compliance is required from children910). It has been suggested that children with GD should receive long-term antithyroid medications, such as methimazole (MMI), which are essential for achieving complete remission. It would therefore be mandatory to predict the response to initial treatment with MMI in such children. Consequently, we conducted this study to identify factors associated with response to initial treatment with MMI in children with GD.
Materials and methods
1. Study population and setting
We conducted the current retrospective, single-center study in 100 patients who had been diagnosed with hyperthyroidism at our medical institution, during a 20-year period ranging from January 1, 1993, to December 31, 2013.
Inclusion criteria for the study were as follows: (1) age was below 18 years, (2) symptoms of GD, (3) past history of taking MMI, and (4) available follow-up data to normalized state of thyroid-stimulating hormone (TSH), free thyroxine (fT4), triiodothyronine (T3).
Exclusion criteria for the study were as follows: (1) loss of follow-up before normalized state of TSH, fT4, T3, (2) other thyroid diseases (e.g., Hashimoto's thyroiditis or papillary carcinoma), (3) past history of taking propylthiouracil (PTU).
We, therefore, enrolled a total of 44 children with GD (n=44) in the current study, which was approved by the Institutional Review Board of our medical institution (approval number: CUH 2015-06-050-002). Informed consent was waived because of the retrospective nature of the current study.
2. Diagnostic criteria and treatment protocol
Diagnosis of GD was confirmed for children presenting with symptoms of hyperthyroidism, such as tachycardia, excessive sweating, weight loss or no weight gain, and elevated serum T3/fT4 levels accompanied by a decreased serum TSH. We treated our clinical series of patients with MMI twice or three times a day11), at a dose of 0.5±0.2 mg/kg/day.
3. Patient evaluation and outcome measures
We analyzed baseline characteristics, such as age, sex, family history of thyroid diseases and comorbidities, through a retrospective analysis of medical records. In addition, we also evaluated changes in serum levels of TSH, T3, fT4, antimicrosomal antibody (AMA), thyroglobulin, thyroid-stimulating immunoglobulin (TSI), and antithyroglobulin antibody (ATA). Furthermore, we analyzed the correlation of normalization time of TF with sex, age at diagnosed state, and family history of thyroid diseases.
To analyze normalization time of thyroid hormones, we divided our clinical series of patients into the following 2 groups: the lower AMA group (serum AMA levels≤100 U/mL) and the higher AMA group (serum AMA levels>100 U/mL).
4. Thyroid function tests
We performed thyroid function (TF) tests, for which we measured serum levels of fT4 (normal range, 11.5–23 pmol/L) and TSH (normal range, 0.17–4.05 mIU/mL) using chemiluminescence immunoassay, based on Luminex xMAP technology (LINCOplex kit; Luminex Corp., Austin, TX, USA). TSI (normal range, 0–10 U/L) and T3 (normal range, 0.78–1.82 ng/mL) were assayed using the rat TSH (rTSH) Biotrak assay system (Amersham Int., Bucks, UK), whereas thyroglobulin (normal range, 0–50 ng/mL) was measured using a commercial reagent set (Dynotest Tg-plus; Brahms Diagnostica, Berlin, Germany). AMA (normal range, 0–0.3 U/mL) was measured using the MBL ELISA kit immunofluorescence antibody assay (RayBiotech, Inc., Norcross, GA, USA) and ATA (normal range, 0–0.3 U/mL) was also measured using the MBL ELISA kit immunofluorescence antibody assay (RayBiotech, Inc.).
5. Patient assignment
We divided our clinical series of patients into the following four age groups: <7 years old, 7–12 years old, 13–15 years old, and 16–18 years old.
6. Statistical analysis
All data was expressed as mean±standard deviation. We performed independent sample t-tests and an analysis of variance to identify correlations between the normalization time and demographic variables. For a post hoc analysis, we used Scheffé method. Statistical analysis was done using the IBM SPSS ver. 18.0 (IBM Co., Armonk, NY, USA). A P-value of <0.05 was considered statistically significant.
Results
1. Baseline characteristics
Our clinical series of patients was composed of 10 boys (22.8%) and 34 girls (77.2%), showing a male-to-female ratio of 1:3.4. The mean age at diagnosis was 12.0±3.2 years.
In our series, the most common symptoms were palpitations (23 cases, 52.3%), goiter (17 cases, 38.6%), weight loss (15 cases, 34.1%), heat intolerance (10 cases, 22.7%), and opthalmopathy (9 cases, 20.5%). In addition, observed comorbidities included 2 cases of diabetes and one case of seizure.
2. Normalization time of TF markers
1) Normalization time of TF depending on sex, age, and family history of thyroid diseases
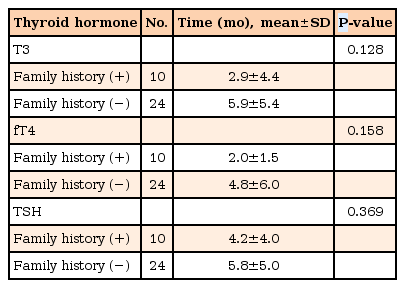
As shown in Table 1, normalization of TF was shorter in girls compared with boys. However, these differences were not statistically significant. In addition, when we analyzed normalization time of TF, this was longer in the group of 16–18 years old compared with other age groups but did not reach statistical significance (Table 2). Furthermore, a family history of thyroid diseases did not affect the normalization time of TF (Table 3).
2) Normalization time of thyroid hormones depended on the AMA
As shown in Table 4, normalization time of T3 was significantly shorter in the higher AMA group compared with that in the lower AMA group (2.53 months vs. 6.18 months) (P<0.05). There was no significant difference in normalization time of fT4 and TSH between the higher and lower AMA groups.
3) Normalization time of thyroid hormones is independent of other TF markers
Normalization of TF did not significantly correlate with other variables such as thyroglobulin, TSI, and ATA. The normal range groups of thyroglobulin, TSI and ATA had shorter normalization time of TF than the increased groups, however they had no significant difference. Normalization time of TF depending on age was not significantly different in each factors. But the patients with less than 7 years old showed the shortest normalization time and the group of 16–18 years old had the longest normalization time among the 4 age groups. Normalization time of TF depending on sex and family history of thyroid diseases was not significantly different in both the normal range group and the increased group of thyroglobulin, TSI, and ATA.
Discussion
Hyperthyroidism is a disease involving hyperactivity of the thyroid gland. Excessive secretion of thyroid hormones can produce symptoms of thyrotoxicosis. In children, the incidence of hyperthyroidism is lower than that of hypothyroidism6). The major cause of hyperthyroidism is GD, and other causes include neonatal thyrotoxicosis, thyroiditis, or McCune-Albright syndrome12). In most pediatric and pubescent patients, symptoms of hyperthyroidism are easily detectable but TF tests are required to confirm diagnosis13). The diagnosis is confirmed when TF shows a decrease in TSH levels and an increase in T4 and T3 levels commensurate with the patient's age14). The treatment methods used include medicines to directly reduce the excessive production and secretion of thyroid hormones, surgical methods, and radioisotope therapy. However, these all have the potential to cause complications1516). Because of antithyroid drugs, the low remission rate, complications, and decreased longitudinal compliance, several studies contended that radioactive iodine therapy or thyroidectomy is needed91718).
Of all the treatment methods, antithyroid drugs should remain the first-line therapy for the treatment of thyrotoxicosis in children313). PTU and MMI, which are antithyroid drugs, interfere with the production of thyroid hormones alleviating symptoms, rather than actually treating the cause of the disease19).
The predictive factors of remission with medical therapy have been studied in children with thyrotoxicosis and have yielded variable results. Allahabadia et al.20) reported that prognosis was poor for younger, male patients. Benker et al.21) stated that patients with high thyroid hormone levels, high thyroid autoantibody levels, and a larger sized thyroid gland prior to treatment had an unfavorable prognosis. Furthermore, pretreatment serum T3 levels were the main factor behind the therapeutic response to MMI in GD. In other studies, the remission rates are higher in those patients with low AMA levels at diagnosis than in those patients who have high serological titers22). AMA can be used as a diagnostic method and high titers of AMA in hyperthyroidism patients represent the immune-mediated disease process. Fine needle aspiration cytology evidence of lymphocytic infiltration in patients with hyperthyroidism is well known323).
Therefore, we assumed that the AMA level before treatment might correlate with the degree of TF responsiveness. Indeed, we found that there was a relationship between the T3 recovery time and the AMA level at diagnosis. The higher AMA titer group developed a normalized T3 earlier than the lower AMA titer group. Consequently, we think that more studies are required to truly ascertain treatment progression in GD patients with higher AMA titers before treatment.
Kim et al.24) showed that prepubertal patients had more severe symptoms and complications than pubertal ones at diagnosis but this conflicts with other studies2526). We were concerned about the relationship between age or other factors at diagnosis, and the normalization time of TF. We found a tendency for fT4 and TSH normalization times to be longer in those more than 16 years old, although there was no statistical correlation. Moreover, the normalization time of T3, fT4, and TSH were not significantly different by sex or family history of thyroid disease.
Our results have several limitations. First, our research was carried out retrospectively and at a single center institution. Second, almost half of the patients dropped out from the original cohort. These drop-out patients could introduce a form of selection bias. Third, we were not able to analyze confounding factors such as drug compliance.
Therefore, serum AMA titers at diagnosis could be used as a predictive factor for the degree of response to initial treatment with MMI in children with hyperthyroidism. To confirm this observation, there is a requirement for further large-scale and multi-institution studies in the future to evaluate the treatment effects of antithyroid drugs in a larger number of children and adolescent patients.
Notes
Conflict of interest: No potential conflict of interest relevant to this article was reported.