Phthalate exposure and childhood obesity
Article information
Abstract
Phthalates are commonly used as plasticizers and vehicles for cosmetic ingredients. Phthalate metabolites have documented biochemical activity including activating peroxisome proliferator-activated receptor and antiandrogenic effects, which may contribute to the development of obesity. In vitro and in vivo studies suggest that phthalates have significant effects on the development of obesity, especially after prenatal exposure at low doses. Although few studies have examined the effects of phthalate on obesity development in humans, some work has shown that phthalates affect humans and animals similarly. In this paper, we review the possible mechanisms of phthalate-induced obesity, and discuss evidence supporting the role of phthalates in the development of obesity in humans.
Introduction
Between the late 1970s and the early 2000s, the prevalence of obesity among Korean children and adolescents rapidly increased nearly 10 folds1). Although the rate of obesity has been leveled off, it remains prevalent particularly in boys2). In general, the increased prevalence of obesity is attributed to overeating, a sedentary life style, and genetic susceptibility. Although high-calorie fast foods and soft drinks are easily available, and people spend more time participating in sedentary activities, such as watching television or using a computer, these factors are insufficient to explain the huge increase in obesity during the 20th century3). In 2002, Baillei-Hamilton4) proposed that the global obesity epidemic was caused by exposure to endocrine disrupting chemicals (EDCs), and demonstrated that increased production of industrial chemicals coincided with increased obesity in the Unites States. A subset of EDCs that promote weight gain and obesity are referred to as "obesogens"5). Obesogens may cause obesity in several ways including disruption of critical lipid metabolism pathways to promote adipogenesis and fat storage, the alteration of the metabolic set point to induce positive energy balance, or increasing appetite5). Indeed, there is evidence showing a positive associations between obesogen levels, including phthalates, and body weight or body mass index (BMI) in children and adults.
Phthalates are diesters of 1,2-benzenedicaraboxylic acid (phthalic acid) and are used to increase the softness and flexibility of plastic products and as vehicles for fragrance in cosmetics. They are widely found in a variety of household products or personal care products, including building materials, shower curtains, children's toys, food packaging, and medical devices. Human exposure to phthalates can occur through ingestion of contaminated food and water, dermal contact, inhalation of polluted air, and parental exposure from medical devices6). Several in vivo and in vitro studies suggest that phthalates may promote obesity through antiandrogenic effects, antithyroid hormone activities, and/or activation of peroxisome proliferator-activated receptors (PPARs). Recently, human studies have been performed to study the association between phthalate exposure and obesity. Children are known to be more vulnerable to environmental exposure to phthalates, as compared to adults, because of their hand-to-mouth activity, larger surface area to weight ratio, and enhanced metabolic rate. As a result, there have been concerns that phthalates may promote childhood obesity in recent years.
In this paper, we review the possible mechanisms by which phthalate might influence the development of obesity, and discuss evidence from human studies suggesting an association between phthalate exposure and obesity-related biomarkers.
Diester phthalates and their potential sources of exposure
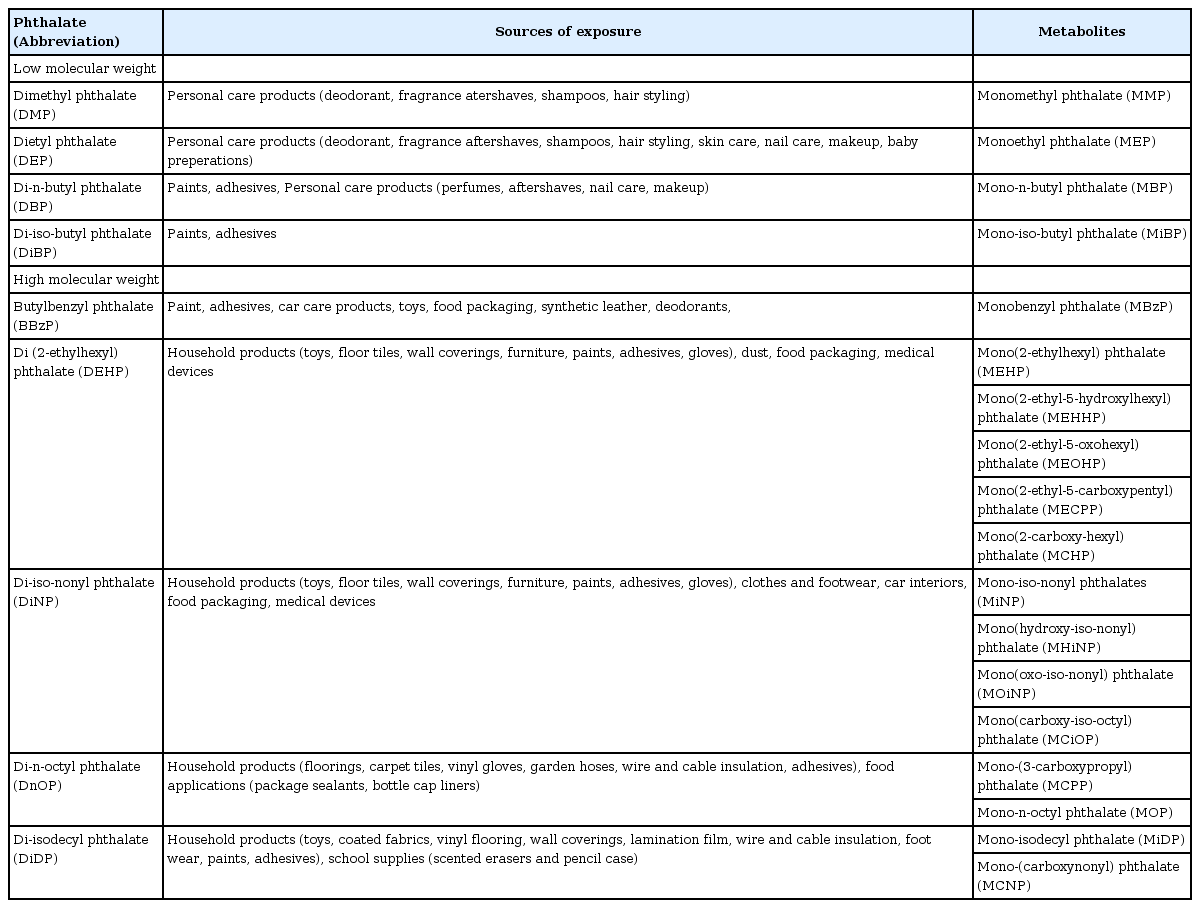
Phthalates have been used as plasticizers since the 1930s, and are currently used as additives in various consumer products (Table 1). The global consumption of phthalates is estimated to be several million tons per year7). High molecular weight (HMW) phthalates, such as di-2-ethylhexyl phthalate (DEHP) and diisononyl phthalate (DiNP) are used primarily in the manufacture of polyvinyl chloride (PVC) plastics for food packaging, building materials, and medical devices. Low molecular weight (LMW) phthalates, such as diethyl phthalate (DEP) and butylbenzyl phthalate (BBzP) are typically used in the manufacture of personal care products (e.g., perfumes, lotions, cosmetics, shampoo), paints, and adhesives. Phthalates are continuously emitted from PVC and plastic materials, resulting in contamination of indoor air, house dust, or food6,7). As a result, the primary methods of HMW phthalate exposure are ingestion of contaminated food or dust, or parental exposure. In contrast, the primary methods of LMW phthalate exposure are inhalation or dermal contact.
Metabolism of phthalates
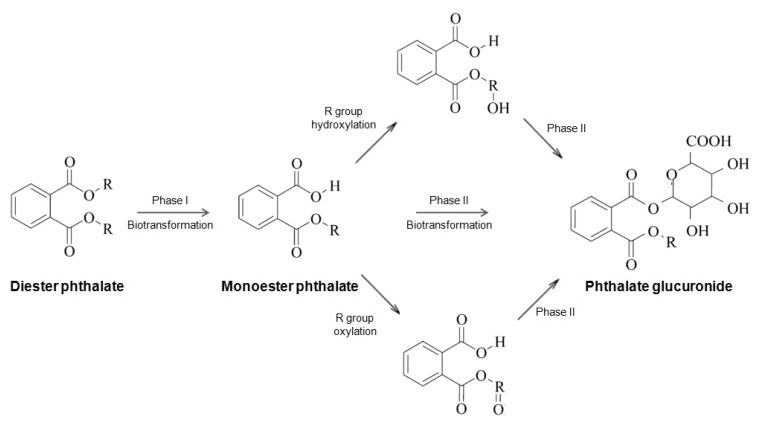
Phthalates are rapidly metabolized and excreted in urine and feces after exposure. Fig. 1 demonstrates the metabolism of phthalates. In phase I hydrolysis, diester phthalates are hydrolyzed by esterases and lipases in the intestine and parenchyma to their respective monoester phthalates8). LMW phthalates are primarily excreted in urine and feces as a monoester, without further metabolism. In contrast, HMW phthalates are further metabolized from monoesters through hydroxylation or oxidation, to produce a number of oxidative metabolites. The oxidative metabolites of phthalates are excreted in urine within 24 hours of exposure. Alternatively, oxidative metabolites can undergo phase II conjugation to form hydrophilic glucuronide conjugates, which are excreted in urine rapidly8). Hydrolytic monoester phthalates can be measured in blood, urine, breast milk, and feces for use as the biomarkers of exposure to the corresponding phthalate diesters. Urinary phthalate metabolites are the most useful biomarkers, as they are relatively easy to collect and their levels in a single sample reflect the exposure to phthalates over several weeks or months9,10). The major biomarker of phthalates with short alkyl chains, such as di-n-butyl phthalate (DBP) and BBzP, are their monoesters in urine7). However, in the case of DEHP and DiNP, which are further metabolized from their primary monoesters and yield numerous oxidative metabolites, exposure must be estimated by taking the sum of primary and secondary metabolites in urine11). When daily phthalate intake was estimated in children using urinary phthalate biomarkers, DEHP was the most abundant phthalate, followed by DBP, di-iso-butyl phthalate, DEP and BBzP12).
Plausible mechanisms of phthalates effects on obesity
PPARs serve as metabolic sensors for various lipophilic hormones, fatty acids, and fatty acid metabolites, thereby controlling adipocyte proliferation and differentiation5). PPARα is highly expressed in liver, heart, skeletal muscle, gonads, and brown adipose tissue, where mediates peroxisome proliferation and stimulate fatty acid β-oxidation13,14,15). PPARα activators exert a variety of metabolic actions, depending on to the species, gender, dose, and timing of exposure. High doses DEHP protected adult mice from diet-induced obesity by promoting fatty acid oxidation and catabolic metabolism by activating PPARα16). In contrast, in mice expressing human PPARα, exposure to DEHP promoted fat accumulation and exacerbated obesity. Further, fetal exposure to low doses of mono(2-ethylhexyl) phthalate (MEHP) significantly increased the body weight of male offspring at postnatal day 60, whereas these effects were not evident in female offspring17). In rodents, phthalate monoesters, including MEHP and mono-n-butyl phthalate, are responsible for deformation of the male reproductive tract and dysfunction of both Leydig and Sertoli cells, resulting in decreased testosterone/androgen production and impaired spermatogenesis13,18). Importantly, phthalates do not interact with androgen receptors directly; rather their anti-androgenic effects are mediated through PPARα13,18). The antiandrogenic effects of phthalates have also been demonstrated in infants and adults19,20). As decreased androgen activity induces obesity, the anti-androgen effect through PPARα may be a possible mechanism of phthalate-induced obesity.
PPARγ is mainly expressed in adipose tissue, It plays a number of key roles including regulating the differentiation of adiopocytes and fat accumulation/storage in the adipose tissue. Additionally, PPARγ improves insulin sensitivity21). PPARγ agonists, such as thiazolidinediones, are potent insulin sensitizing agents used to control hyperglycemia in type 2 diabetes. However, their side effects include weight gain, which limits their usage in obese patients. Some phthalate monoesters, such as MEHP, mono-iso-nonyl phthalates, and mono-isodecyl phthalate act as PPARγ agonists, thereby promoting differentiation and lipid accumulation in 3T3-L1 cells, similar to thiazolidinediones22,23). Therefore, it is likely that phthalates exert an adipogenic effect though the activation of PPARγ. However, few in vivo animal studies have been performed to assess the effects of phthalate on PPARγ and adipogenesis17).
Another possible mechanism by which phthalates might promote obesity is through the disruption of thyroid function, which plays a key role in the regulation of energy balance and metabolism. There is some evidence that thyroid function plays a role in the regulation of BMI, as small changes in thyroid-stimulating hormone (TSH) or thyroxine levels within the normal range can cause measurable differences in resting energy expenditure in chronic hypothyroidism patients, and slight elevation of serum TSH levels are associated with both weight gain over 5 years and obesity in a population study24,25). In rodent studies, exposure to DEHP lowered plasma thyroxine and decreased iodide uptake of thyroid follicular cells26,27). Recent human studies have also demonstrated possible effects of phthalate exposure on thyroid function in children and adults28,29,30,31).
Finally, the "thrifty phenotype" resulting from exposure to undernourished fetal environment and EDCs could be one of plausible mechanisms by which phthalates promote obesity32). Epigenetic changes, induced by a suboptimal fetal environment, may result in increased uptake and conservation of nutrients, and predispose individuals to obesity and other metabolic disorders32). Epidemiological studies provide evidence that maternal malnutrition during pregnancy and subsequent low birth weight is associated with obesity later in life33,34,35). In rodent studies, maternal exposure to DBP or DEHP during the gestational period have been reported to decrease birth weight in offsprings36,37). Studies regarding of the effect of phthalate exposure on preterm delivery and/or fetal growth in humans are limited and conflicting. Some studies suggested that there is a positive association between fetal phthalate exposure and premature delivery or lower birth weight38,39,40), but other studies failed to show a significant relationship41,42). Prospective investigations are needed to reveal the validity of the hypothesis that phthalate exposure results in low birth weight and subsequent obesity.
Phthalate exposure and obesity development in human
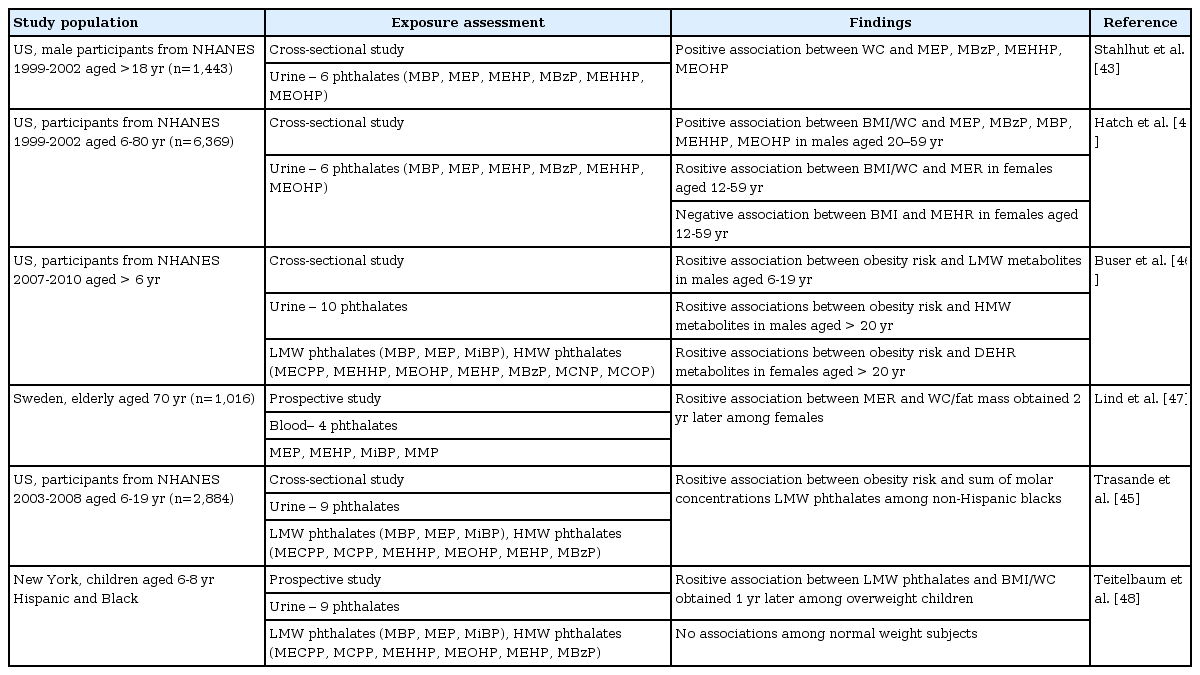
Table 2 presents the results of human studies investigating the effects of phthalate exposure on obesity. Most epidemiologic studies examining the association between phthalate exposure and obesity have been based on the data from the National Health and Nutrition Examination Survey (NHANES)43,44,45,46). Regarding adulthood obesity, Stahlhut et al.43) demonstrated a positive association between urinary monoethyl phthalate (MEP), monobenzyl phthalate, mono(2-ethyl-5-hydroxylhexyl) phthalate, and mono(2-ethyl-5-oxohexyl) phthalate and waist circumference (WC) in male adults, using data from NHANES 1999-200243). Using the same data, Hatch et al.44) showed a positive association between urinary MEP and both BMI and WC in female adults. Recently, a study from NHANES 2007-2010 found that HMW phthalates were associated with an increased risk of obesity in male adults, while DEHP phthalates were associated with increased obesity in females46). A prospective study from Sweden investigated serum phthalate metabolites in elderly subjects (70 years), and measured their body composition by dual-energy X-ray absorptiometry (DXA) two years later. In this study, serum mono-isobutyl phthalate levels were significantly correlated with increased BMI, WC, total fat mass and trunk fat mass by DXA in females, but not in males47).
Emerging evidence suggest childhood exposure to some phthalates also may increase the risk of obesity. In a study of Hatch et al.44), BMI and WC increased with urinary MEP concentrations among female girls in the United States. Two recent studies using data from NHANES found that urinary levels of LMW phthalates were associated with higher odds for obesity in children and adolescents45,46). A prospective cohort study also found that urinary LMW phthalate metabolite concentrations were positively associated with BMI in overweight children. However, no associations were reported among all the total subjects or normal weight subjects alone48). The health effects of phthalate exposure appear to be complex, as they are dependent on several factors, such as the time of exposure, level of exposure, type of phthalates, and other environmental/genetic factors of the individuals.
Conclusions
Many in vitro studies indicate that phthalates are likely obesogens, promoting obesity via several mechanisms, including activation of PPARs, antithyroid effects, and epigenetic modulation. The fetal period appears to be a critical window for exposure, and differential effects are observed depending on the dose of phthalates received and gender. Recent human studies have examined the possible effects of phthalate exposure on the development of obesity, although most of them are cross-sectional and short-term prospective studies. Although the random concentrations of phthalate metabolites have good reproducibility, large-scaled longitudinal study including measures at different life ages is needed to establish the impact of phthalate exposure on the obesity epidemic.
Acknowledgments
This work was supported by grant 11162KFDA701 from the Korea Food & Drug Administration in 2011.
Notes
No potential conflict of interest relevant to this article was reported.