Earlier re-evaluation may be possible in pediatric patients with eutopic congenital hypothyroidism requiring lower L-thyroxine doses
Article information
Abstract
Purpose
The incidence of congenital hypothyroidism (CH) has increased in several countries. Lower cut-off in screening programs have led to an increase in the proportion of transient hypothyroidism (TH) cases diagnosed, leading to debate on the associated clinical and economic impact. This study aimed to identify factors that would allow discrimination between TH and permanent CH (PH) in patients with a eutopic thyroid gland.
Methods
Sixty-six patients with CH from 3 different hospitals were studied: 26 cases of TH, and 40 cases of PH. Laboratory findings and clinical parameters were analysed in 56 patients with eutopic thyroid gland.
Results
Initial serum thyroid stimulating hormone levels and L-thyroxine dose at 12 and 24 months of age were significantly higher in PH than TH patients with a eutopic thyroid gland. The area under the curve for the 12-month and 24-month dose for the prediction of TH in eutopic CH was 0.799 (95% confidence interval [CI], 0.678-0.919; P<0.001) and 0.925 (95% CI, 0.837-1.000; P<0.001), respectively. The optimum 12-month and 24-month dose in predicting TH is 3.25 µg/kg (12-month: sensitivity, 87.1%; specificity, 68.0%; 24-month: sensitivity 93.5%, specificity 88%).
Conclusion
Infants with CH requiring lower L-thyroxine doses (<3.25 µg/kg) are likely to have TH, and thus might be re-evaluated at 12 months or 24 months rather than 3 years of age.
Introduction
In developed countries, the introduction of screening programs for congenital hypothyroidism (CH) has enabled effective detection of this condition in neonates. In response to screening, the prevalence of CH has increased, particularly for patients with a eutopic (normally located) thyroid gland and a mild elevation in thyroid-stimulating hormone (TSH; hyperthyrotropinemia)1). The reasons for this may be related to lower screening thresholds compared to initial values, allowing for more effective detection of CH, and an increase in the number of premature infants as a result of development of reproductive technologies2,3,4). Thyroid hormones play an important role in early neurodevelopment, and thus the early treatment of hypothyroidism is crucial1,5,6). Although the long-term outcomes of mild hypothyroidism or hyperthyrotropinemia are not yet fully understood, low screening thresholds and early intervention can mean unnecessary or over-treatment of hyperthyrotropinemia or transient hypothyroidism (TH). Permanent CH (PH) usually results from thyroid dysgenesis (athyreosis, ectopic thyroid), whereas the underlying aetiologies of TH are not clear; however, suggested factors include iodine deficiency or excessive iodine intake, penetration of maternal thyroid auto-antibodies, preterm delivery, or immaturity of thyroid iodine organification7,8,9). Current guidelines recommend treatment in patients less than 2 weeks of age if their venous free thyroxine (fT4) concentration is lower than average, or if their venous TSH concentration is higher than 20 mU/L, this age threshold is lower than that of previous guidelines1,10). Generally, further investigations on aetiology are not performed at initial diagnosis, and re-evaluation at 3 years of age is recommended; thus, it is possible that patients with TH are for treated longer than necessary1,10). A number of studies have been conducted to determine how best to discriminate between cases of TH and PH, when treatment should be stopped, and when reevaluation can be performed11,12,13,14). Hypothyroidism due to thyroid aplasia and/or ectopic thyroid require L-thyroxine lifelong. Therefore if the infants are documented to have thyroid dysgenesis, L-thyroxine treatment is required. However, in case of hypothyroid infants with eutopic thyroid gland, definitive criteria have not yet been suggested.
In this study, we investigate the differences between TH and PH in patients with a eutopic thyroid gland, and attempt to identify factors enabling their distinction.
Materials and methods
1. Patients and methods
Two hundred and sixty-eight children, who underwent a thyroid function test (TFT) owing to suspected CH (elevated TSH on neonatal screening test or prolonged jaundice) at St. Vincent's Hospital, Bucheon St. Mary's Hospital, and Yeouido St. Mary's Hospital of The Catholic University of Korea between January 2004 and April 2014 were enrolled in this study. Medical records were reviewed retrospectively, and patients were confirmed as having primary CH (TSH>10 mU/L and/or fT4 <0.7 ng/dL in venous blood). L-thyroxine treatment was started immediately in infants diagnosed with primary CH, at a dose of 10-15 µg/kg. One hundred and thirty-one patients were confirmed as having primary CH and treated with L-thyroxine. Of these, 29 patients were excluded owing to incomplete medical records, and a further 36 were excluded as the follow-up period was less than 3 years. Sixty-six patients with CH with follow-up at least 3 years after initial diagnosis were included in the final analysis (Fig. 1).
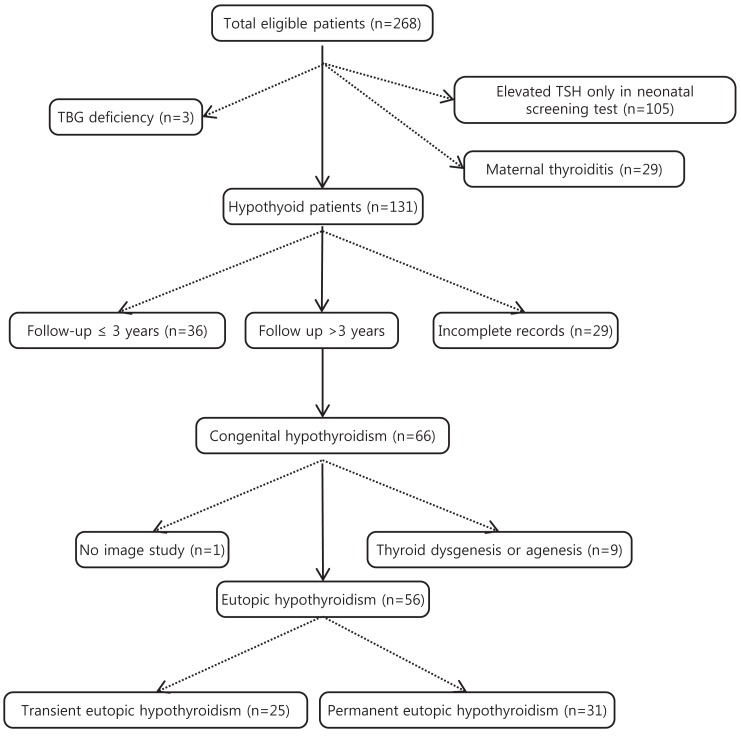
Flow diagram of the study population with congenital hypothyroidism. TBG, thyroxine binding globulin; TSH, thyroid-stimulating hormone.
Patients were treated with L-thyroxine until 3 years of age and re-evaluated after 4 weeks of discontinuing medication; followup was carried out at least 4 years from the initial diagnosis. Thyroid ultrasonography or a Technetium-99 (Tc-99m) thyroid scan was performed either at diagnosis or at the 3-year follow-up. Either thyroid ultrasonography and/or a thyroid scan were performed to identify patients with thyroid dysgenesis. Thyroid scans were conducted on 27 patients and ultrasonography on 65; however, one patient with TH underwent neither. Nine patients with PH had thyroid agenesis or dysplasia: 4 of these had thyroid agenesis, 2 had agenesis with an ectopic sublingual thyroid gland, and 3 had thyroid hypoplasia. Ultrasonography showed that 56 patients had a eutopic thyroid gland. These were subsequently divided into 2 groups, TH (transient eutopic hypothyroidism: TSH<5 mU/L) and PH (permanent eutopic hypothyroidism: TSH>10 mU/L). Follow-up was carried out every 1-3 months for patients with hyperthyrotropinemia (TSH, 5-10 mU/L). These patients were all defined as having either PH or TH after the follow-up period. The TH group contained patients for whom treatment could be discontinued at the 3-year follow-up, and the PH group contained patients for whom treatment continued (Fig. 1, Table 1).
All patients were monitored 1 month after L-thyroxine discontinuation, and every 6 months thereafter. Serum triiodothyronine (T3), fT4, and TSH levels were measured by chemiluminescent microparticle immunoassay with the Architect i2000 analyser (Abbott, Abbott Park, IL, USA). Reference values were as follows: T3. 0.58-1.9 ng/mL; fT4, 0.7-1.79 ng/dL; and TSH, 0.35-4.95 µIU/mL. L-thyroxine dose per kilogram body weight was calculated at the onset of treatment, at 12 months of age (12M dose), and at 24 months of age (24M dose).
This study complied with the recommendations of the Declaration of Helsinki and was approved by the Institutional Review Boards of St. Vincent's Hospital, Bucheon St. Mary's Hospital, and Yeouido St. Mary's Hospital of The Catholic University of Korea.
2. Statistical analysis
Statistical analyses were performed with PASW Statistics ver. 18.0 (SPSS Inc., Chicago, IL, USA). Continuous variables were expressed as the mean±standard deviation. Continuous data were analysed using the Student t-test and Mann-Whitney test, and categorical variables using the chi-square test or Fisher exact test. A receiver operating characteristic (ROC) curve was designed to estimate the optimum cut-off value in dosage per kilogram for indicating PH. Sensitivity and specificity for this optimum value in the estimation of PH were calculated. The optimum cut-off value was defined as the highest Youden index [(specificity+sensitivity)-1]. P-values lower than 0.05 were considered statistically significant.
Results
1. Analysis of patients with a eutopic thyroid gland
Of the 56 study patients, 25 patients were assigned to the TH group and 31 patients to the PH group. None of the patients had associated malformations. Baseline clinical and laboratory values for patients with eutopic thyroid gland are presented in Table 1. The ratio of female to male patients in both groups was similar as 1.1:1. Low birth weight infants accounted for 8.0% of the TH group and 19.4% of the PH group. Premature infants was 12.0% of the TH group and 12.9% of the PH group. Initial serum TSH levels were higher in the PH group than the TH group (89.4±67.1 mU/L vs. 60.5±78.3 mU/L, P=0.038) (Table 1). Initial serum fT4 and T3 levels were not significantly different between groups. Both the 12M dose and 24M dose were higher in the PH group than the TH group (12M dose: 4.5±1.5 µg/kg vs. 3.1±1.1 µg/kg, P<0.001; 24M dose: 4.2±0.8 µg/kg vs. 2.5±1.0 µg/kg, P<0.001) (Table 1).
2. Estimation of the optimum cut-off dose of L-thyroxine for prediction of TH in patients with a eutopic thyroid gland
The ROC curves predicting TH using initial TSH level, 12M dose, and 24M dose are shown in Fig. 2. The area under the curve (AUC) for initial TSH for the prediction of TH was 0.662 (95% confidence interval [CI], 0.518-0.806; P=0.039), and the optimum cut-off value for initial TSH was 28.4 mU/L (sensitivity, 80.6%; specificity, 52%) (Fig. 2). The AUC for the 12M dose for the prediction of TH was 0.799 (95% CI, 0.678-0.919; P<0.001), and the optimum cut-off value, based on the maximum Youden index, was 3.25 µg/kg (sensitivity, 87.1%; specificity, 68.0%) (Fig. 2). 12M dose of the highest sensitivity (100%) was 1.9 µg/kg, and that of the highest specificity (100%) was 5.55 µg/kg. The AUC for 24M dose for the prediction of TH was 0.925 (95% CI, 0.837-1.000; P<0.001), The optimum cut-off value of 24M dose was 3.25 µg/kg (sensitivity, 93.5%; specificity, 88%). Additionally, 24M dose of the highest sensitivity (100%) was 2.8 µg/kg and that of the highest specificity (100%) was 5.0 µg/kg.

Receiver operating characteristic (ROC) curve of initial serum TSH, L-thyroxine dose at 12 months of age (12M dose), and 24 months of age (24M dose) reflecting transient congenital hypothyroidism. The cutoff values of 12M dose and 24M dose were 3.25 µg/kg (area under the curve=0.799; 95% CI, 0.678-0.919; P<0.001; sensitivity, 87.1%; specificity, 68.0%) and 3.25 µg/kg (area under the curve=0.925; 95% CI, 0.837-1.000; P<0.001; sensitivity, 93.5%; specificity, 88%), respectively. TSH, thyroid-stimulating hormone; CI, confidence interval.
Discussion
In the present study, the prevalence of TH was 39.4%, which is comparable with that in other recent studies which showed values of 38%-54.9%11,12,15,16,17). Based on this information, more than one-third of CH cases do not require lifelong medication. Therefore, markers that would allow early detection of TH are required.
In this retrospective review of pediatric patients with CH with a eutopic thyroid gland, initial venous TSH concentration, 12M dose, and 24M dose were significantly higher in PH than TH cases. Among these patients, a 12M and 24M dose >3.25 µg/kg indicated a higher chance of developing PH. Current guidelines recommend the administration of 10-15 µg/kg of L-thyroxine after the initial diagnosis of CH, followed by dose titration at 1- to 3-month intervals to maintain a venous TSH concentration within the average range1). Hong et al.11) states that the only difference between PH and TH is the L-thyroxine dose required. They showed that required doses are significantly lower in TH than in PH at 6 months, 12 months, 24 months, and 36 months of age, with doses of 3.3±1.2 µg/kg for TH and 4.8±1.3 µg/kg for PH at 12 months of age. Unuvar et al.12) also suggest that only L-thyroxine dose can be used to distinguish between TH and PH, with values of 2.8±1.19 µg/kg for TH and 4.07±1.88 µg/kg for PH at 12 months of age given. These doses are comparable to those described here; however, their study population contained patients with thyroid dysgenesis, and the authors did not suggest an optimum cut-off dose in predicting TH. Thus, to the best of our knowledge, our study is the first to suggest a cut-off dose of L-thyroxine that would allow discrimination between babies with TH and PH in eutopic CH.
TSH concentrations in venous blood at initial diagnosis were higher for PH than TH in this study. This has also been shown in several other studies12,17,18,19). Lim et al.17) suggest a cut-off TSH level of 34 mU/L (sensitivity, 72%; specificity, 93%) for the prediction of PH. In the present study, cut-off TSH level was 28.4 mU/L with slightly higher sensitivity and lower specificity than that of Lim et al.17). On the other hand, Rabbiosi et al.16) suggest there is no difference in TSH concentration between PH and TH, although initial TSH concentrations might vary depending on when the blood sample was drawn. If the sampling was carried out too early, TSH levels may not have been sufficiently elevated. Therefore, TSH concentration at initial diagnosis cannot accurately predict the severity of CH.
Several reports have suggested that preterm and low birth weight infants have a higher tendency to have TH20,21,22,23,24). Preterm, low birth weight infants are usually critically ill and vulnerable to neurodevelopmental disability. Thus, even if their thyroid dysfunction is transient, L-thyroxine treatment is necessary. In the present study, 6 of the 9 preterm or low birth weight infants had PH. However, our results were not significant in predicting TH or PH.
Otherwise, the prevalence of thyroid dyshormonogenesis was 75% (31 from 40 patients with PH). It is various from 31.3% to 76.9% in several studies11,12,17,25). These wide variety of prevalence might be due to the various ethnicity and whether perchlorate discharge test was done or not. We did not conduct perchlorate discharge test to infants with eutopic thyroid gland.
In contrast to past standards, current guidelines recommend early treatment with L-thyroxine (where possible at less than 2 weeks of age) when the venous TSH concentration is >20 mU/L irrespective of fT4 concentration, and "playing safe" by treating with L-thyroxine during early childhood when TSH levels are low (6-20 mU/L) and fT4 levels are normal1). In addition, once treatment is started, re-evaluation is not recommended until 3 years of age or older. This is based on the fact that myelination of the brain is completed by 36-40 months of age26), and that the child may be more co-operative during imaging. Thyroid scans require sedation, intravenous access, and should be carried out at least 5 days after L-thyroxine administration begins, and thus cannot be easily conducted for neonates at the initial diagnosis of CH. However, ultrasonography is not invasive, and can be easily performed for neonates, although it cannot identify dyshormonogenesis. Therefore, we suggest that neonates with a high likelihood of developing CH be treated immediately, and undergo thyroid ultrasonography. If the infants have thyroid dysgenesis, re-evaluation can be delayed until 3 years of age or over. According to the present study, the patients with eutopic thyroid gland requiring L-thyroxine >5.55 µg/kg at 12 months or >5.0 µg/kg have highly suggestive of PH, they should not be decreased the dose or carefully re-evaluated at 3 years of age. Patients requiring lower L-thyroxine doses tend to have TH at the re-evaluation of 3 years of age in some studies16,25). Furthermore, if patients requiring lower than 1.9 µg/kg at 12 months and lower than 2.8 µg/kg at 24 months can be transient hypothyroidism in the present study. These patients could be tapered the dosage of L-thyroxine at 12 months or 24 months easily. In the present study if infants have a eutopic thyroid gland, and L-thyroxine doses <3.25 µg/kg are required at 12 months and 24 months of age, re-evaluation may be performed carefully by decreasing the dose of L-thyroxine by 30% for 2-3 weeks, and rechecking by TFT1). This study has several limitations. It involved a retrospective review of medical records. Although patients from 3 different hospitals were included, the relatively small study population hindered significance in our analyses. In addition, the sensitivity and specificity for the cut-off with the highest Youden index of the 12M and 24M dose in predicting PH were not particularly high; therefore, it cannot be used as a definitive standard. Further long-term studies on the benefits of L-thyroxine treatment in TH in relation to neurodevelopment are also required.
The results of this study suggest that infants with CH who require lower L-thyroxine doses by 12 months and 24 months of age are likely to have TH. Therefore, infants with CH requiring lower L-thyroxine doses (3.25 µg/kg at 12 months and 24 months) may be re-evaluated at 12 months or 24 months rather than 3 years of age by decreasing the dose of L-thyroxine.
Notes
No potential conflict of interest relevant to this article was reported.
