Denosumab use in osteogenesis imperfecta: an update on therapeutic approaches
Article information
Abstract
Osteogenesis imperfecta (OI) is an inherited skeletal disorder that leads to bone fragility and multiple fractures. Given advances in the genetic understanding of existing phenotypes and newly discovered mutations, therapeutic management of OI has become challenging. Denosumab, a monoclonal antibody that inhibits the interaction between the receptor activator of nuclear factor kappa B ligand (RANKL) and its receptor RANK, has been approved to treat postmenopausal osteoporosis and emerged as an important therapy for malignancies and other skeletal disorders, including pediatric skeletal conditions such as OI. This review summarizes information about denosumab therapy in OI by exploring its mechanisms of action, main indications, and safety and efficacy. Several case reports and small series have been published about the short-term use of denosumab in children with OI. Denosumab was considered a strong drug candidate for OI patients with bone fragility and a high risk of fracture, particularly for patients with the bisphosphonate (BP)-unresponsive OI-VI subtype. The evidence for denosumab's effects in children with OI indicates that it effectively improves bone mineral density but not fracture rates. A decrease in bone resorption markers was observed after each treatment. Safety was assessed by tracking the effects on calcium homeostasis and reporting side effects. No severe adverse effects were reported. Hypercalciuria and moderate hypercalcemia were reported, suggesting that BPs be used to prevent the bone rebound effect. In other words, denosumab can be used as a targeted intervention in children with OI. The posology and administration protocol require more investigation to achieve secure efficiency.
Highlights
· Therapeutic management of osteogenesis imperfecta has become increasingly challenging because of advances in the genetic understanding of existing phenotypes and newly discovered mutations.
· The genetic cause of OI-VI and the understanding of its pathogenesis suggest that the RANK/RANKL pathway could be targeted with the RANKL antibody denosumab as an individual translational therapeutic approach.
· Bone mineral density, fracture rate, bone turnover markers, growth, and mobility were evaluated to assess the efficacy of denosumab.
· Calcium homeostasis and side-effect reporting were used to assess denosumab safety. Hypercalciuria and moderate hypercalcemia were reported, suggesting that bisphosphonates be used to prevent the bone rebound effect.
Introduction
Osteogenesis imperfecta (OI) is a rare genetic skeletal condition caused by a group of heritable connective tissue disorders that lead primarily to bone fragility and multiple fractures [1]. In most cases, heterozygous mutations in the COL1A1 or COL1A2 gene cause OI skeletal symptoms by affecting the synthesis and structure of collagen type I [2]. During the past decade, several mutations in other genes have been identified as causes of OI, and it is now understood to be a genetically polymorphic condition with a broad range of phenotypes [3].
In the 2019 revised classification of genetic skeletal disorders, OI is part of the "decreased bone density group." [4] The Online Mendelian Inheritance in Man classification lists 5 types of OI and several new subtypes that are distinguished and defined by the form of inheritance, causal gene, and clinical spectrum [4,5]. The 5 main OI types are: non deforming type with persistently blue sclerae (OI-I), perinatal lethal form (OI-II), progressively deforming type (OI-III), moderate form (OI-IV), and calcification of the interosseous membranes and/or hypertrophic callus (OI-V). Systemic cardiac, neurological, and respiratory disorders and hearing impairment have also been reported as contributors to the morbidity and mortality of OI [5].
Given advances in the genetic understanding of existing phenotypes and newly discovered mutations, therapeutic management of OI has become increasingly challenging. During the past few years, clinical trials and prospective therapies have been studied continuously, but conclusive evidence about their effectiveness is still inadequate [6].
On the assumption that an increase in bone mineral density (BMD) would reduce bone pain and the fracture rate, antiresorptive drugs such as bisphosphonates (BPs) have been widely used in OI management [7]. Although BPs have been shown to increase BMD, the latest data do not indicate an improvement in the clinical status [8]. Moreover, given the possible long-term side effects of BPs in children with OI, such as the possibility that the drugs will be stored in the skeleton for years [9], various other treatment modalities have been explored [7].
Denosumab, a monoclonal antibody that inhibits the interaction between the receptor activator of nuclear factor kappa B ligand (RANKL) and its receptor RANK, was approved in 2010 for the treatment of postmenopausal osteoporosis [10]. Due to its antiresorptive properties, denosumab has also emerged as an important therapy for malignancies and other skeletal disorders, including some pediatric conditions such as OI [11]. By inhibiting RANKL to RANK binding, denosumab decreases the differentiation of preosteoclasts, which reduces the number and activity of osteoclasts and slows bone resorption.
This report reviews the main therapeutic features of denosumab in OI in terms the: (1) mechanisms of action, (2) indications, (3) dosage and administration, (4) efficacy, and (5) tolerance and side effects.
Search strategy
We performed a literature review in the PubMed and Google Scholar databases using the following sets of keywords: "Osteogenesis imperfecta," "Management," "Therapy," and "Denosumab." Studies published in or before January 2023 that included OI patients who were treated with denosumab were eligible. All types of study designs restricted to human subjects were included. Articles not in English or French and with no translation could not be part of the study.
Mechanisms of action
In children, bone growth occurs through a bone modeling and remodeling process that alternates between bone formation by osteoblasts and bone resorption by osteoclasts [12]. Bone formation occurs on the outer periosteal surface and increases bone size, and the marrow cavity is expanded by resorption, which sculpts the inner surface of the bone [13,14].
Several genetic mutations causing OI are involved in the molecular pathways of bone modeling and remodeling. The most common ones occur in the COL1A1 and COL1A2 genes, which encode the α1 and α2 chains of type I collagen, which is the most prevalent protein in pre-mineralized bone matrix [6,15]. In OI, defects in collagen structure and the disruption of osteoblast differentiation lead to cortical thickness abnormalities and altered trabecular microarchitecture [16]. As a result, reduced bone mass and increased bone fragility affect bone quality and create a high risk of fractures [15]. Furthermore, it has been shown that in OI-III and OI-IV, the rates of bone turnover are abnormally increased, which contributes to bone fragility [16,17].
Therapeutic strategies to treat OI target the bone shaping cycle by using antiresorptive drugs to inhibit osteoclasts or drugs that promote bone formation through osteoblasts. BPs have been the antiresorptive drugs most widely used in OI management, and intravenous BPs such as pamidronate and neridronate have been commonly used in patients with severe clinical OI symptoms [18,19]. However, patients with skeletal symptoms have often shown a poor response, and fractures have persisted despite BP administration [20,21]. Additionally, BPs might not be as effective in OI-VI as in other types of OI. Patients with OI-VI commonly show a poor response to BPs, probably due to the impossibility of binding the unmineralized osteoid, which is particularly high in OI-VI. That impairs the ability of BPs to induce osteoclastic apoptosis and inhibit bone resorption [20].
Furthermore, because BPs are stored in the bone, their long-term safety for children has been extensively debated, raising doubts about the feasibility of their long-term use [8,22,23]. For example, prolonged BP use can put patients at an increased risk of atypical proximal femoral fractures and jaw osteonecrosis [8,22,23].
Another factor in the high risk of fractures caused by OI is an increase in bone mineralization. Although this finding is often misunderstood, too much bone mineralization makes bones brittle [24-26].
RANKL acts on osteoclasts through a different mechanism than BPs [27]. Highly expressed by osteoblasts, RANKL is a paramount regulator of bone resorption. After binding to RANK on the surfaces of osteoclasts and preosteoclasts, RANKL promotes osteoclast activity, which increases bone resorption [28]. Binding between RANKL and RANK is blocked by soluble osteoprotegerin (OPG), which is produced by osteoblasts and promotes calcium and bone homeostasis [29,30].
Denosumab is a fully human monoclonal antibody to RANKL. By blocking the binding between RANKL and RANK and mimicking the inhibiting effects of OPG, denosumab can decrease the differentiation of preosteoclasts, thereby reducing bone resorption and increasing bone mass. In suppressing bone resorption, this mechanism of action seems to be similar to that of BPs. Moreover, preclinical studies of denosumab efficacy in animal models suggest that it has a long-term effect on periosteal bone formation [31].
Indications
In 2010, denosumab was approved for the treatment of postmenopausal osteoporosis in patients with an increased risk of fracture or who have failed or are intolerant to BP therapy [32,33]. In the randomized, double blind, FREEDOM study, denosumab (60 mg once every 6 months) was associated with a decline in serum markers, an increase in BMD, and low fracture incidence in patients with postmenopausal osteoporosis [34]. Since then, denosumab has been widely and safely used in adults for the treatment of primary and secondary osteoporosis, bone metastases, hypercalcemia of malignancy refractory to BPs, and giant-cell tumors (because RANKL is highly expressed by abnormal stromal cells) [10].
To date, pediatric data about denosumab use remain sparse. The pharmacological properties of denosumab in children are unknown [10]. However, it has recently been used to treat pediatric skeletal conditions, particularly those that affect the RANKL pathway, and shown potentially promising results. Several case reports and small series have been published about the short-term use of denosumab in children with different conditions: OI, juvenile Paget disease [35], fibrous dysplasia [36], central giant-cell granuloma [37], metastatic giant-cell tumor [38], and spinal aneurysmal bone cysts [39]. It has been used to decrease bone turnover and prevent the growth of some bone tumors in children.
OI is the most common cause of primary osteoporosis in children [40]. The goals of treatment include decreasing the fracture rate, reducing pain, and promoting growth and mobility to maintain a high quality of life. Several research groups have considered denosumab to be a strong drug candidate for OI patients with bone fragility and a high risk of fracture, even those with BP-unresponsive OI [21,41-47].
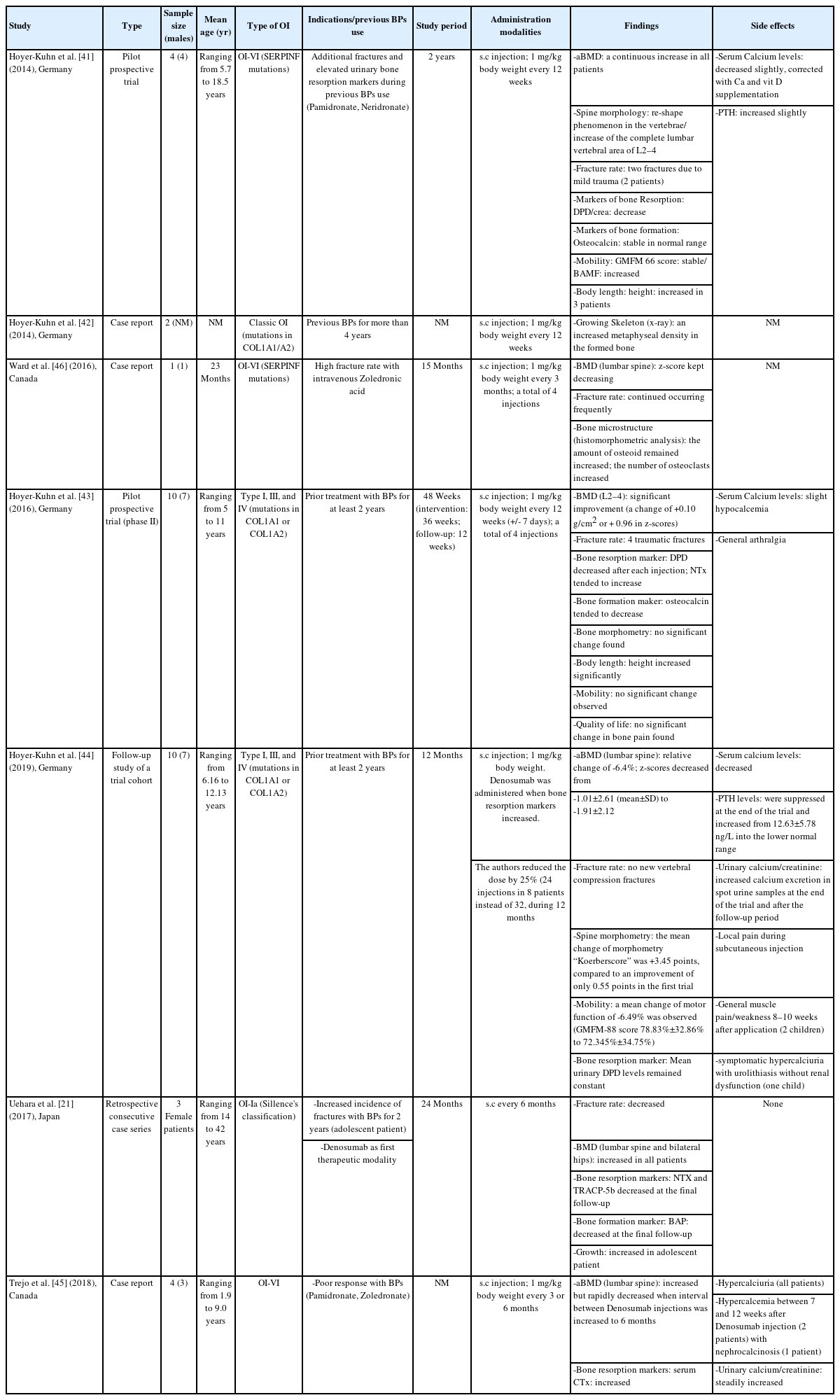
Many children with OI are currently treated with intravenous BPs regardless of their underlying mutation. OI-VI is an autosomally recessive form caused by SERPINF1 mutations and is histologically characterized by prominent unmineralized osteoid and a poor response to BPs [48]. Semler et al. [19] reported the first use of denosumab in 4 children with a fracturing and deforming form of OI-VI in 2012, and Hoyer-Kuhn et al. [41] published a pilot prospective trial 2 years later. Trejo et al. [45] also reported using denosumab in 4 pediatric patients with OI-VI in 2018 (Table 1). Deciphering the genetic cause and pathogenesis of OI-VI led the above-mentioned authors [19,41,45] to target the RANK/RANKL pathway using the RANKL antibody denosumab as an "individual translational therapeutic approach."
This alternative was considered after patients showed a poor response to therapy with intravenous BPs, including pamidronate, neridronate [49], and zoledronate [45]. Despite BP use with physiotherapy and rehabilitation, additional fractures occurred, and almost all the children depended on a wheelchair. Urinary levels of deoxypyridinoline (DPD), an indicator of osteoclastic activity, also remained consistently high [47].
Ward et al. [46] reported the case of a 23-month-old child from northern Canada. OI-VI was diagnosed with an iliac bone biopsy that showed features typical of OI-VI. Denosumab was initiated after intravenous BP administration. An iliac bone sample obtained after 5 denosumab injections showed an increase in the number of osteoclasts in trabecular bone. This report offered histological evidence of the rapid effect of denosumab in a child with OI-VI [46].
Uehara et al. [21] reported a retrospective case series of 3 female patients (2 adults and an adolescent) with classical OI-Ia who were treated with denosumab to assess its effect on bone fragility. In the adolescent patient, intravenous BPs were used before denosumab, but the fracture rate remained persistently high. Hoyer-Kuhn et al. [42] reported on 2 children with OI caused by mutations in COL1A1/A2 who responded poorly to BPs and were switched to denosumab after 4 years. That report assessed the effects of denosumab on the growing skeleton. In 2016, Hoyer-Kuhn et al. [44] also conducted a 48-week, open-label, pilot study of the safety and efficacy of denosumab in 10 children with OI-I, OI-III, and OI-IV and at least 2 years of prior BP treatment. In addition, a retrospective evaluation of an individualized biomarker-associated treatment regime with denosumab was performed for 1 year after their participation in the initial study [44].
Dosage and administration
Few data are available on the pharmacokinetics and pharmacodynamics of denosumab in children. Zheng et al. [50] assumed that bone modeling and growth in children characterized their increased skeletal metabolism, highly affecting the dosage and dosing intervals of RANK, RANKL, and OPG. Due to the decreased body weight and surface area of children, those authors deemed that monoclonal antibody dosing could be reduced from the adult regime [50]. The amount of RANKL produced by children and the effects of the disorders denosumab is being given to treat on its production seem to be unknown. In adults, denosumab clearance depends on the amount of available RANKL [51]. In children, a low concentration of the RANKL antibody relative to the concentration of RANKL would lead to rapid elimination of the antibody [50].
According to Uehara et al. [21], a subcutaneous injection of denosumab was administered to OI-Ia patients every 6 months. Table 1 summarizes the doses and intervals of denosumab administration in previous studies [41,45-47]. A protocol of calcium and vitamin D supplementation was instituted in some cases due to common vitamin D depletion [41,47]. According to Hoyer-Kuhn et al. [44], after 1 year of treatment, denosumab administration was assigned to shorter intervals (minimum period: 10 weeks), based on the recurrence of pain and the increase in urinary bone resorption markers (assessed as urinary DPD/creatinine) 8 weeks after injection. In addition, Semler et al. [47] noticed that a 3-month interval might be too long for patients with OI-VI and suggested that an 8-week interval might be more appropriate to ensure a constant suppression of osteoclastic bone resorption.
Hoyer-Kuhn et al. [41] reported that the appropriate duration of treatment and frequency of injections cannot yet be defined. They suggested that the treatment should be continued until the end of growth, following the regime used for BPs.
Efficacy
The data available from small clinical trials that used denosumab to treat OI demonstrate its efficacy in terms of clinical, biological, and radiological features. The primary clinical outcomes included BMD and fracture rates. Biomarkers of bone resorption and bone formation were secondary outcomes. In addition, studies have focused on bone morphometry and microstructure, growth, and mobility (Table 1).
Li et al. [52] conducted a systematic review of the effects of denosumab on children with OI, including 2 pilot prospective trials and one case report involving a total of 15 children. Those authors concluded that the evidence for denosumab's effects on children with OI was inconclusive. The main limitations of the included studies were the absence of control groups, short-term observations, small sample sizes, and different management of OI-VI, which impaired their validity and reproducibility [52].
1. BMD and fracture rates
During the 2-year study period in the report from Uehara et al. [21], all OI-Ia patients had slightly increased lumbar and hip BMD, and no bone fractures occurred. In the adolescent patient, denosumab treatment started at the end of growth, when the fracture rate tends to decrease in most patients irrespective of treatment. Denosumab is, therefore, unlikely to be solely responsible for the fracture-free interval. Furthermore, the modest increase in BMD was explained by high BMD values at baseline [21].
According to the pilot prospective trial (phase II) conducted by Hoyer-Kuhn et al. [43], BMD (L2–4) improved significantly, with a change of +0.10 g/cm2 (+0.96 in z-score) after 12 weeks of follow-up. Long-bone fractures due to trauma were observed during the treatment period. During the follow-up year, the mean relative change in lumbar areal BMD was −6.4%, and the lumbar spine areal BMD z-scores decreased from −1.01±2.61 (mean±SD) to −1.91±2.12 [44]. Those authors concluded that bone mass at the end of the trial was still higher than at the start.
In their case report about a 23-month-old child, Ward et al. [46] assessed the occurrence of multiple vertebral compression fractures, as well as lower extremity ones. The BMD (lumbar spine) z-score kept decreasing in this patient also. Histomorphometric analysis of the bone microstructure showed a constant increase in the amount of osteoid. The number of osteoclasts also increased substantially.
According to Semler et al. [47], during a follow-up time of five to 33 weeks, one patient suffered a fracture of the humerus bone after a mild trauma. However, no data about BMD were provided. Thus, denosumab appears to effectively improve BMD but not fracture rates.
2. Bone turnover markers
The reported bone turnover markers differed from one study to another. As bone formation markers, Uehara et al. [21] evaluated bone alkaline phosphatase and urinary N-terminal telopeptide of type-I collagen (NTX) before and after denosumab administration. On the other hand, they reported only tartrate-resistant acid phosphatase 5b as a marker of bone resorption. All markers were improved by denosumab.
Some authors measured urinary levels of DPD, a marker of osteoclast activity, throughout the treatment period and normalized it to urinary creatinine (Crea) levels (DPD/Crea) [41,43,47]. Urinary DPD values after denosumab administration indicated the suppression of bone resorption and a decrease in NTX values. According to Hoyer-Kuhn et al. [41], a DPD decrease increased areal BMD. Those authors considered that to be supporting evidence of denosumab efficacy. However, during the follow-up period, the DPD/Crea levels slowly increased, reaching pretreatment levels 6–8 weeks after denosumab administration and suggesting that the effect of denosumab in suppressing bone resorption was reversible [47]. Similarly in osteoporotic adults treated with denosumab and BPs [32], Semler et al. [47] interpreted a decrease in the serum level of pro-collagen-1-C-peptide as a marker of osteoblastic activity.
As a marker of bone formation, osteocalcin levels remained in the normal range or tended to decrease [43]. Given the increase in NTX levels, Hoyer-Kuhn et al. [43] suggested that suppression of bone metabolism might outweigh bone formation. However, constant growth in the patients was observed based on growth velocity data, measurement of vertebral height, and extremity x-rays [42].
3. Growth
According to Uehara et al. [21], no clinical reports have des cribed the effects of denosumab or RANKL inhibition on growth in children. However, the first short-term trials of denosumab use in children are available. Wang et al. [53] reported radiological and histological changes after denosumab in a child with fibrous dysplasia whose legs had both been amputated. Continued epiphyseal activity was observed during and after treatment, as was the reversal of bone turnover suppression after denosumab discontinuation, suggesting that denosumab does not have significant adverse effects on growth. Furthermore, Uehara et al. [21] did not identify a growth disturbance in their adolescent case. The patient's height increased after the first administration of denosumab. Additionally, a long-lasting effect of denosumab has been demonstrated by an increase in the metaphyseal density of bone between drug injections, indicating that longitudinal growth continued [42]. Furthermore, in their pilot prospective trial (phase II), Hoyer-Kuhn et al. [43] reported that height increased significantly among the OI-VI children treated with denosumab. The follow-up study also demonstrated a significant increase in the mean height and a constant z-score during the 2-year observation period [44]. These findings suggest that no disturbance in growth was caused by denosumab, and an overall increase in the treated children’s height was mostly observed.
4. Mobility
Because it is largely affected by OI, mobility has been one of the main outcomes in previous studies. Semler et al. evaluated mobility at the start of denosumab treatment using the Brief Assessment of Motor Function scale [47,54]. However, they did not report that denosumab had an effect because all patients depended on a wheelchair before treatment began. In the pilot prospective trials conducted by Hoyer-Kuhn et al. [41,43,44], mobility, assessed by the gross motor function measurement, neither improved nor deteriorated. In the study of Uehara et al. [21], mobility was not assessed because it was not restricted in any of the patients .
5. Tolerance and side effects
Denosumab has significant inhibitory effects on bone turnover. Its safety was addressed using bone turnover markers and side-effect reporting. In phase 3 trials, the bone turnover markers in denosumab-treated subjects were lower than those in placebo- and BP-treated subjects [10]. However, in the available clinical trials that included OI patients treated with denosumab, most authors reported no or few adverse events, particularly among children. Uehara et al. [21] observed mild or moderate side effects, such as hypocalcemia, during denosumab treatment. Similarly, in clinical trials involving children with OI-VI, no treatment discontinuation based on unexpected side effects was reported [41,47]. The injections were well tolerated without any allergic reactions or disruption in vital signs. Slight hypocalcemia was observed after each injection, but it was considered to be an indicator of reduced osteoclastic activity to resorb bone and release calcium [41]. This was compensated for with oral calcium supplementation. No secondary hyperparathyroidism was observed. However, data concerning urinary calcium excretion were not reported [41,47]. Those authors concluded that a strong effect of an antiresorptive drug such as denosumab can be expected in children, whose bone turnover is especially high. They assumed that treatment until the end of puberty might decrease the rebound effect after the end of therapy [41].
The pilot prospective trial of Hoyer-Kuhn et al. [43] showed slight hypocalcemia related to denosumab and general arthralgia and muscle pain in most patients, possibly related to treatment. In their follow-up study, they reported that one severely affected patient developed symptomatic hypercalciuria [44]. Urolithiasis was diagnosed without renal dysfunction or any further complications.
Trejo et al. [45] reported a case series in which they assessed hypercalcemia and hypercalciuria during denosumab treatment in children with OI-VI. All subjects developed hypercalciuria during denosumab therapy. In addition, hypercalcemia with nephrocalcinosis was found in 1 patient only 7 weeks after the preceding denosumab injection. On the other hand, Hoyer-Kuhn et al. [41] reported that the antiresorptive effect of a denosumab injection seemed to last only 6 to 8 weeks. That motivated them to decrease the interval between denosumab injections from the original 12 weeks to a minimum of 10 weeks. According to Trejo et al. [45], the probability of developing hypercalcemia during denosumab treatment might vary depending not only on the diagnosis but also on individual characteristics such as age, growth rate, and the activity of bone metabolism. The long-term antiresorptive effect provided by BPs would be suitable once denosumab is discontinued to prevent the rebound effect and rapid bone loss with intermittent hypercalciuria/hypercalcemia during denosumab therapy [45].
Conclusions
This review summarizes current knowledge about denosumab use in OI. Denosumab is a promising antiresorptive drug that could be used as a targeted intervention in children with musculoskeletal disorders that affect the RANKL pathway. Further studies should evaluate the effects of this treatment approach on mobility, bone density, and fracture rates and focus on potential adverse effects related to bone turnover rebound and serum calcium homeostasis. The posology and administration intervals also require more investigation to ensure effectiveness and consistent osteoclast inhibition.
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Funding
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author contribution
Conceptualization: FM, HLF; Data curation: FM, WH; Methodology: FM, HLF, DBN, DK, KM, WH; Visualization: FM; Writing - original draft: FM, HLF, KM, WH; Writing - review & editing: FM, HLF, DBN, DK, KM, WH