Short-term effect of growth hormone treatment in childhood leukemia survivors with growth hormone deficiency
Article information
Abstract
Purpose
Survivors of childhood leukemia are at risk of growth impairment due to intensive chemotherapy and radiation treatments. This study investigated the auxological and biochemical characteristics of childhood leukemia survivors diagnosed with growth hormone deficiency (GHD) and the changes in these parameters after 1 year of growth hormone (GH) treatment.
Methods
A total of 24 children diagnosed with GHD after leukemia treatment was analyzed. Clinical and biochemical data were collected retrospectively at leukemia diagnosis, GHD diagnosis, and 1 year after GH treatment. Standard deviation score (SDS) was calculated based on the age- and gender-adjusted population.
Results
Of the 24 children included in this study, 19 received GH treatment. The median age at GHD diagnosis was 12.3 years, and the median delay in bone age was 1.46 years. Height SDS decreased from -0.69 at leukemia diagnosis to -2.58 at GHD diagnosis (P<0.001). The change in height SDS with and without GH for 1 year was 0.35 and -0.21, respectively (P=0.044). In regression analyses, higher height SDS at GHD diagnosis and a smaller decrease of the height SDS between leukemia and GHD diagnoses were positively correlated with height SDS after GH treatment.
Conclusions
GH treatment could be beneficial and safe for improving height in childhood leukemia survivors with GHD. Height SDS at GHD diagnosis and reduction of height SDS between leukemia and GHD diagnosis could be potential factors in predicting the therapeutic effects. Close auxological monitoring is recommended for any childhood leukemia survivors who experience posttreatment height decline.
Highlights
· This study investigated the auxological and biochemical characteristics of childhood leukemia survivors diagnosed with growth hormone deficiency (GHD) and the changes in these parameters after 1 year of growth hormone (GH) treatment. The change in height standard deviation score with and without GH for 1 year was 0.35 and -0.21, respectively (P=0.044).
Introduction
Acute leukemia is the most common childhood malignancy. As cancer cure rates for childhood-onset malignancies have improved, the number of cancer survivors has increased [1]. Over the last several decades, improvements in survival rates for childhood leukemia can be attributed to intensive multiagent regimen chemotherapy and central nervous system prophylaxis [2-4]. However, these multiagent chemotherapies and radiation therapy increase the risk of various late-onset chronic health conditions called "late effects," impacting quality of life [5]. Two of the late effects reported among survivors were considerable growth retardation and decreased final adult height [6,7].
Diagnosis of growth hormone deficiency (GHD) in childhood is an intricate process that involves a comprehensive assessment of various clinical and auxological features combined with biochemical tests and radiological evaluation. GHD can appear as an isolated issue or as a component of multiple pituitary hormone deficiencies [8]. Although guidelines and traditional clinical criteria for GHD determine a peak growth hormone (GH) value <10 ng/mL in provocation tests, it is important for clinicians to create a diagnosis based on the combination of available data given the lack of a diagnostic gold standard [8]. In children with GHD, GH treatment can improve final adult height and body composition [9,10]. The starting dose of GH ranges from 25–43 µg/kg/day, but this can be exceeded during puberty [11,12]. The purpose of GH treatment is to improve quality of life by normalizing the deviated aspects of growth and attaining timely puberty and body composition throughout adult life [13].
Endocrine diseases can develop for decades after cancer treatment, emphasizing the importance of lifelong surveillance in childhood cancer survivors [7]. GHD in cancer survivors has characteristics similar to those seen in the noncancer population. These include increased fat mass, poor lipid profile, increased cardiovascular morbidity, decreased bone density, impaired quality of life, and psychosocial problems [7,14,15]. In patients treated with cranial radiation therapy, GHD is the most common endocrine late effect [16]. Guidelines recommend using the same provocative tests to diagnose GHD and the same treatment regimens for both childhood cancer survivors and the noncancer population [7]. Although there have been reports on GH treatment for GHD in cancer survivors, only a few studies have reported statistical benefits and treatment responses among leukemia survivors [17-19].
Therefore, in this study, we investigated the characteristics of children with GHD diagnosed after treatment for leukemia and examined the auxological and biochemical changes after 1 year of GH treatment.
Materials and methods
1. Subjects
Between January 2010 and January 2020, 1,456 patients were diagnosed with leukemia, including acute lymphoblastic leukemia (ALL), acute myeloid leukemia (AML), and chronic myeloid leukemia (CML) at the Department of Pediatrics, Seoul St. Mary's Hospital (College of Medicine, Catholic University of Korea, Seoul, South Korea). After being treated for leukemia, patients' heights were measured at every regular endocrine outpatient follow-up, and GH stimulations were performed when growth retardation with height less than 3 percentile was identified. Patients who had not concluded leukemia treatment due to recurrence or unstable conditions were excluded from the GHD evaluation. A final total of 24 patients was diagnosed with GHD and enrolled in this study.
The median age of leukemia diagnosis in the enrolled patients was 4.26 years, the height standard deviation score (SDS) was -0.69, and the body mass index (BMI) SDS was -0.09. Fifteen children had ALL, while 9 had AML. All children were treated with chemotherapy, 17 received radiation treatment, and 21 underwent hematopoietic stem cell transplants. Nineteen patients received steroids for graft-versus-host disease (GVHD) after transplantation. Nine patients received additional treatment for leukemia recurrence before being diagnosed with GHD (Table 1). Nineteen patients received GH treatment. Of the 5 untreated patients, 3 denied GH treatment due to the risk of adverse effects and the burden of daily injections. The other 2 patients did not show up at outpatient follow-up to begin treatment after GHD diagnosis.
2. Data collection and laboratory measurements
The medical charts of the enrolled subjects were reviewed retrospectively to collect demographic, auxological, and biochemical data (1) at leukemia diagnosis, (2) at GHD diagnosis, and (3) 1 year after GHD diagnosis or treatment.
Anthropometric data extracted from the medical records included sex, chronological age, height, weight, BMI, bone age, GH dose (mg/kg body weight/wk), maximum GH serum level in stimulation tests, and biochemical markers. A Harpenden Stadiometer (Holtain Ltd., Crymych, UK) was used to measure height to the nearest 0.1 cm using the average of 3 measurements. The weight was measured using the same scale to the nearest 0.1 kg at every outpatient follow-up. The SDS of height, weight, and BMI were collected from the 2017 Korean Children Growth Chart [20].
Biochemical markers including serum insulin-like growth factor-1 (IGF-1), insulin-like growth factor-binding protein-3 (IGFBP-3), cortisol, glucose, hemoglobin A1c, insulin, homeostatic model assessment of insulin resistance (HOMA-IR), free T4, thyroid-stimulating hormone (TSH), total cholesterol, triglyceride, high-density lipoprotein cholesterol, and low-density lipoprotein cholesterol levels were analyzed. All blood samples were collected in the morning after overnight fasting. The SDS values of IGF-1 and IGFBP-3 were adjusted for sex and age [21]. Bone age was measured using the Greulich-Pyle method with simple radiographic images of the left hand and wrist. The mean values determined by 1 radiologist and 2 pediatric endocrinologists were used for the analysis.
3. Diagnosis of GHD
Two GH stimulation tests were conducted in the morning after overnight fasting at 24-hour intervals with administration of levodopa (Myung In Pharm, Seoul, Korea; <15 kg: 150 mg; 15–35 kg: 250 mg; >35 kg: 500 mg) or arginine (Green Cross Well Being, Bundang, Korea; 0.5 g/kg, max 30 g). Blood samples were collected at the time of administration of the stimuli and at 30, 60, 90, and 120 minutes after stimulation to measure peak serum GH level. Patients with a peak GH level <10 ng/mL after stimulation with 2 stimulants were classified as having GHD [8].
4. Statistical analysis
Data are presented as median and interquartile range (25% and 75%, respectively). Statistical analyses were performed using IBM SPSS Statistics ver. 24.0 (IBM Co., Armonk, NY, USA). Changes in height SDS, weight SDS, and BMI SDS between diagnosis and 1-year posttreatment were analyzed using the Mann-Whitney U-test. The effects of GH treatment were compared between the 19 treated patients and 5 untreated patients using Mann-Whitney U-tests. The effects of each variable on height SDS after 1 year of treatment were examined using linear regression. Statistical significance was set at a P-value <0.05.
5. Ethical statement
This study protocol was reviewed and approved by the Institutional Review Board of Seoul St. Mary's Hospital (approval number: KC22RISI0017). The requirement for written informed consent was waived due to the retrospective nature of the study.
Results
1. Characteristics of patients at GHD diagnosis
The auxological and biochemical data of patients diagnosed with GHD after leukemia treatment are shown in Table 2. At GHD diagnosis, the median age was 12.3 years and the bone age was delayed by approximately 1.5 years. At the time of GHD diagnosis, the height SDS was -2.58, which was a decrease in -1.85 from the SDS value at the time of leukemia diagnosis. The median levels of the biochemical markers at GHD diagnosis were as follows: average peak GH during stimulation tests, 5.09 ng/mL; free T4, 1.33 ng/dL; TSH, 2.56 mIU/L; and cortisol, 6.47 µg/mL. Regarding glucose metabolism, the median values for fasting glucose, hemoglobin A1c, fasting insulin, and HOMA-IR were 88 mg/dL, 5.1%, 11.22 µU/mL, and 2.84, respectively. The median values of IGF-1 SDS and IGFBP-3 SDS were -1.39 and -1.69, respectively. Nineteen patients received GH treatment, and the median starting dose was 0.24 mg/kg/wk. Height SDS decreased significantly from -0.69 at leukemia diagnosis to -2.58 at GHD diagnosis in 24 patients (P<0.001). However, the decrease in BMI SDS from -0.09 at leukemia diagnosis to -0.68 at GHD diagnosis was not significant (P=0.06).
2. Difference in height between patients with and without GH treatment (Fig. 1)
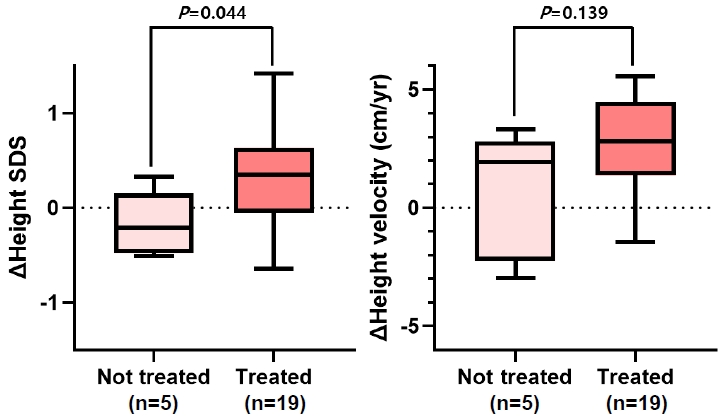
Difference in height status between growth hormone-treated patients and untreated patients. SDS, standard deviation score.
The change in height SDS decreased to -0.21 in the latter group, whereas it increased to 0.35 in the former group (P=0.044). In terms of change in height velocity (cm/yr), from baseline at GHD diagnosis, the median height velocity change at 1 year after GHD diagnosis was lower (1.94 cm/yr) in patients who did not receive GH treatment compared with those who received treatment (2.82 cm/yr). However, the difference was not significant (P=0.139).
In the comparison of height velocity in the 19 treated patients, the median value of height velocity increased from the basal (4.21 cm/yr) to the posttreatment (6.53 cm/yr) level with statistical significance (P<0.001) (Supplementary Fig. 1).
3. Biochemical changes after 1 year of GH treatment
Comparisons of the levels of biochemical markers before and after 1 year of GH treatment in a group of 19 patients are shown in Table 3. During the treatment period, there was no recurrence of leukemia or occurrence of other tumors. Significant increases were observed in IGF-1 SDS (-1.40 to -0.07) and IGFBP-3 SDS (-1.54 to -0.73), whereas no significant changes were seen in the levels of thyroid hormone and cortisol. Fasting blood glucose, HbA1c, and insulin levels exhibited an increasing trend, while total cholesterol and triglyceride levels decreased.
4. Analysis of height SDS after 1 year of GH treatment in prepubertal children
In 10 of the treated patients, bone age (BA) (male≥12, female≥10) was in puberty, while the median Tanner stage changed from 2 to 3 after 1 year of GH treatment. In 9 prepubertal children, regression analysis of height SDS after 1 year of GH treatment was performed using factors at leukemia and GHD diagnoses (BA, male<12, female<10) (Table 4). Age, height SDS, type of leukemia, duration, and dose of steroid treatment were not significant factors at the time of leukemia diagnosis. A smaller difference between chronic age and bone age, a larger height SDS at GHD diagnosis, and a smaller decrease in height SDS between the diagnoses of leukemia and GHD were positively correlated with height SDS after GH treatment.
Discussion
In this study, we analyzed the characteristics of patients diagnosed with GHD after leukemia treatment and evaluated the effects of GH treatment for 1 year. We determined that GH treatment was associated with improved height status but not with apparent risk of tumor recurrence, impaired lipid profiles, or metabolic syndrome. A larger height SDS at the time of GHD diagnosis and less decrement in height SDS between leukemia and GHD diagnoses were positively correlated with a larger height SDS after treatment. The incidence and severity of height decrement can be varied due to differences in chemotherapeutic regimens and inclusion of radiation therapy [22]. Height SDS at GHD diagnosis and decrement in height SDS between leukemia and GHD diagnoses are the consequence of complex effects of the risk factors of height deficit. Therefore, a larger height SDS and smaller decrement in height SDS could imply less harm from these risk factors. Based on these findings, GH treatment is a better prognostic factor in the less disadvantageous state.
To the best of our knowledge, only a few studies have assessed the efficacy of GH treatment in leukemia patients because of the rarity of the patient group. Several previous studies have reported better growth outcomes in GH-treated children. In a study on 15 children with leukemia who developed radiation-induced GHD, Didi et al. reported that the height velocity improved from 5.4 to 7.6 cm/yr after 1 year of GH treatment [18]. Consistent with these findings, Adan et al. reported lower mean height loss (0.6 SDS) following GH treatment in 17 children diagnosed with GHD after receiving cranial irradiation for ALL compared with 15 untreated children with subnormal GH status (1.7 SDS) [19]. In a systematic review of 29 observational studies on GH treatment in childhood cancer survivors, Tamhane et al. [23] reported a significant positive correlation between GH treatment and height gain. In line with these previous studies, we observed a significant increase in height SDS in the GHtreated group. Unlike the study by Adan et al. [19], the control group in our study had confirmed GHD. Overall, our findings are consistent with those of previous studies, demonstrating that GH treatment improves height in pediatric leukemia survivors with GHD.
Adult ALL survivors diagnosed with GHD are at a higher risk of adverse cardiovascular and diabetes risk profiles than those without GHD and tend to have more components of metabolic syndrome [24]. Improvement in systolic cardiac function and reduced prevalence of metabolic syndrome were reported after GH replacement in these patients [25]. However, GH treatment did not improve the metabolic indicators in our study, which could be due to the small number of patients with abnormalities in baseline tests and short-term follow-ups as well as the relatively younger age of the patient group.
The prevalence of GHD in the general population is estimated at approximately 1:4,000 to 1:10,000 [26,27]. However, the prevalence of radiation-induced GHD varies depending on the population, follow-up time, and type of provocation test. One study reported a prevalence of radiation-induced GHD ranging from 29.0% to 39.1% when only strict peak GH cut-off limits were selected [28]. Another study reported 120 cases of GHD among 324 ALL patients (37%) diagnosed over 40 years [29]. In our study, the prevalence of GHD in leukemia patients was approximately 1:60, much higher than in the general population. The higher prevalence in this study is believed to be related to leukemia treatments, such as chemotherapy and radiation treatment. Given the rarity of these conditions, the exact prevalence of GHD in leukemia patients has not been established, and it has been difficult to conduct randomized tests, making our study a valuable contribution to the literature.
Recombinant human GH has been used for more than 30 years, and its indications have gradually expanded. IGF-I is highly dependent on GH and affects its biological action in peripheral tissues. Due to the mitogenic and anti-apoptotic activities of IGF-I, GH treatment for diseases other than GHD has raised safety concerns such as cancer risk [30]. Relationships between elevated circulatory level of IGF-I and slightly increased risk of prostate, breast, lung, and colorectal neoplasms and leukemia have been supported by cumulative evidence from epidemiological studies [31-34]. However, several observational studies through childhood have confirmed that cancer risk is not increased by the therapeutic use of GH [23,35-37]. Consistent with previous studies, during the 1-year follow-up period in this study, there was no leukemia recurrence in children treated for GHD.
The recent guidelines for the European Society of Endocrinology and Pediatric Endocrine Society recommend prospective follow-up of linear growth in childhood cancer survivors, who are likely to have short stature as adults due to radiation treatment or prolonged steroid use. However, these guidelines do not recommend GH treatment without confirmed GHD. GH treatment is recommended for patients with confirmed GHD who have been cancer-free for 1 year. The recommended GH treatment regimen is identical to the one used for the noncancer population [7].
This study has several limitations. First, the power of statistics is inevitably low because of the retrospective nature of the study of a small number of patients. Second, the study subjects, including prepubertal and pubertal children, are not homogeneous. Therefore, the precise efficacy of GH treatment remains unclear. Third, the effects of leukemia treatment regimens, including dosage and duration of chemotherapy, radiation, and hematopoietic stem cell transplantation, on growth impairment in total leukemia patients diagnosed with or without GHD have not been fully investigated. Last, detailed evaluations of adverse drug reactions in GH treatment patients were insufficient.
In conclusion, in patients diagnosed with GHD after childhood leukemia treatment, GH treatment could be beneficial and safe in improving height status without affecting biochemical indicators. The decrease in height SDS between the diagnoses of leukemia and GHD as well as that of height SDS at GHD diagnosis are potential factors that can predict the therapeutic effects of GH. Therefore, close monitoring of auxological parameters is recommended for all childhood leukemia survivors who experience posttreatment height decline to diagnose GHD early. Further studies with larger sample sizes and longer follow-ups are warranted to analyze the risk factors for GHD after leukemia treatment and to determine the predictors of GH treatment efficacy.
Supplementary material
Supplementary Fig. 1 can be found via https://doi.org/10.6065/apem.2244028.014.
Supplementary Fig. 1.
Difference in height velocity between basal and post treatment in 19 treated patients. GH, growth hormone.
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Funding
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data availability
The data that support the findings of this study can be provided by the corresponding author upon reasonable request.
Author contribution
Conceptualization: BKS, MBA; Data curation: CS, MBA; Formal analysis: CS; Methodology: CS, BKS, MBA; Project administration: MBA; Visualization: CS; Writing - original draft: CS; Writing - review & editing: CS, MJJ, SK, JWL, NGC, BC, MHJ, BKS, MBA




