Risk factors of postoperative hypoparathyroidism after total thyroidectomy in pediatric patients with thyroid cancer
Article information
Abstract
Purpose
Hypoparathyroidism (hypoPTH) is the most common complication following thyroidectomy. We investigated the frequency and risk factors of hypoPTH after total thyroidectomy (TT) in pediatric patients with thyroid cancer.
Methods
This retrospective study included 98 patients younger than 20 years who were diagnosed with thyroid cancer after T T during 1990–2018 and followed for more than 2 years at Seoul National University Hospital. HypoPTH was defined as receiving active vitamin D (1-hydroxycholecalciferol or 1,25-dihydroxycholecalciferol) after surgery.
Results
The study included 27 boys (27.6%) and 71 girls (72.4%). The mean age at diagnosis was 14.9±3.7 years. HypoPTH occurred in 43 patients (43.9%). Twenty-one patients (21.4%) discontinued active vitamin D less than 6 months after surgery, while 14 (14.3%) continued active vitamin D for more than 2 years. Tumor multifocality (odds ratio [OR], 3.7 vs. single tumor; P=0.013) and preoperative calcium level (OR, 0.2; P=0.028) were independent predictors of hypoPTH immediately after TT. In addition, age (OR, 0.8; P=0.011) and preoperative calcium level (OR, 0.04; P=0.014) significantly decreased the risk for persistent hypoPTH requiring active vitamin D for more than 2 years.
Conclusions
HypoPTH occurred in 43.9% of pediatric thyroid cancer patients after TT in this study. Among them, one-third of patients continued active vitamin D medication for more than 2 years, which was predicted by young age and low preoperative calcium level.
Highlights
· Hypoparathyroidism is common after total thyroidectomy in pediatric thyroid cancer patients.
· Patients with young age, low preoperative calcium levels and those with multifocal tumor should be monitored intensively for postoperative hypoparathyroidism.
Introduction
Although thyroid malignancy is less common in the pediatric population, the incidence rate of pediatric thyroid cancer has increased worldwide [1-3]. For children with differentiated thyroid cancer, total thyroidectomy (TT) is strongly recommended as the initial surgical approach, and postoperative radioactive iodine therapy (RAIT) is indicated for intermediate- or high-risk patients [4]. Pediatric thyroid cancer presents with a more advanced stage at diagnosis and carries a higher recurrence rate compared to adult thyroid cancer patients [5], but these young patients show a lower mortality rate [6].
Postoperative hypoparathyroidism (hypoPTH) remains the most common complication after thyroid surgery. Most patients recover within a few months, but some must continue active vitamin D medications for permanent hypoPTH [7]. Patients with permanent hypoPTH have increased risk of long-term morbidity in association with renal and cardiovascular diseases [7,8]. The prevalence of postoperative hypoPTH after pediatric thyroid surgery varies from 0%–36.4% [9-19] depending on the indication for surgery, extent of lymph node dissection, or definition of hypoPTH. Although several studies conducted with many pediatric thyroid cancer patients in other countries have revealed risk factors of hypoPTH, including central neck dissection (CND) or extent of surgery [9,11,17], no pediatric study on this subject has been conducted in Korea. Considering the high survival rate and long life-expectancy of pediatric thyroid cancer survivors, efforts to limit risk factors for postoperative hypoPTH are warranted.
In this study, we investigated the frequency and follow-up course of hypoPTH after TT in pediatric patients with thyroid cancer. We analyzed risk factors for postoperative hypoPTH requiring active vitamin D medication and predictors for discontinuing medication within 2 years after thyroidectomy.
Materials and methods
1. Subjects
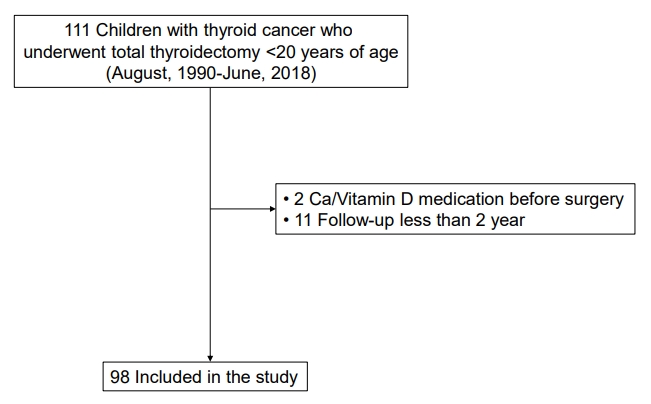
The medical records of 111 patients (<20 years of age) who underwent TT for pediatric thyroid cancer at Seoul National University Children's Hospital between August 1990 and June 2018 were retrospectively reviewed. All patients underwent TT or completion thyroidectomy within 6 months of initial lobectomy or subtotal thyroidectomy. After excluding 13 patients who had taken calcium and/or vitamin D medication before surgery (n=2) and those followed for less than 2 years (n=11), 98 patients were finally included in this study (Fig. 1). The timing of last follow-up was defined as the date of the last outpatient clinic visit without reoperation.
2. Clinic opatholo gical presentation and surgical management
Management and follow-up strategies for thyroid cancer have been described previously [5]. In brief, surgical approaches including TT, subtotal thyroidectomy, or lobectomy have been employed as initial treatment methods in accordance with prophylactic or therapeutic lymph node dissection. RAIT was recommended for patients with large tumor (>1 cm), extrathyroidal extension (ETE), or lymph node and/or lung metastasis. Clinical and treatment information including previous radiation history, type of operation (laparoscopic or open), type of node dissection, and number of autotransplanted parathyroid glands (PTGs) were obtained. Autotransplantation of PTGs into the sternocleidomastoid muscle was performed after frozen-section confirmation when PTG viability was questionable or when a PTG was unintentionally removed or devascularized during surgery [4]. Type of neck dissection was defined according to the 2009 American Thyroid Association consensus statement [20]. In detail, CND included resection of prelaryngeal and pretracheal nodes with at least one paratracheal lymph node, while lateral node dissection (LND) was defined as resection of cervical lymph node levels ll–V. Meanwhile, cases treated by the "plucking" or "berry-picking" method, where only clinically involved nodes rather than the complete nodal group within the compartment were removed [20], were classified as the "other" node dissection group. Pathologic findings including tumor size, multifocality, ETE, TNM staging [21], weight of the thyroid specimen, and presence of PTGs in the specimen were obtained. Since autotransplanted or inadvertently excised PTGs result in reduced function of the parathyroid parenchyma after thyroidectomy, the importance of in situ preserved PTGs has been emphasized [22,23]. Thus, we calculated the Parathyroid Gland Remaining In Situ (PGRIS) score by subtracting the number of autotransplanted PTGs during thyroidectomy and the number of PTGs identified in the pathological specimen from 4 cases, i.e., the total number of PTGs in normal thyroid anatomy (PGRIS score=4 – [autotransplanted PTGs + PTGs in the specimen]).
3. Biochemical assessment and evaluation of postoperative hypoPTH
Perioperative data on calcium, 25-hydroxy vitamin D (25OHD), and intact parathyroid hormone (iPTH) levels were collected. Postoperative calcium level was measured within 12 hours after thyroid surgery with monitoring of hypocalcemic symptoms such as tingling, numbness, and paresthesia. Oral calcium and active vitamin D medication were prescribed when postoperative calcium level was lower than 8.0 mg/dL and/or when a patient complained of hypocalcemic symptoms. Postoperative hypoPTH was defined as receiving active vitamin D (1-hydroxycholecalciferol or 1,25-dihydroxycholecalciferol) supplementation after surgery. Total duration, dose, and type of active vitamin D medications were investigated during the follow-up course. Since all patients who took active vitamin D at 2 years after surgery maintained the medication until the date of last follow-up, patients were classified into hypoPTH (no), hypoPTH (yes, <2 years), and hypoPTH (yes, ≥2 years) groups according to whether they took active vitamin D medication (yes or no) and the duration of medication use (<2 or ≥2 years).
4. Statistical analysis
All statistical analyses were performed using the IBM SPSS Statistics ver. 25.0 (IBM Co., Armonk, NY, USA). Continuous variables were expressed as mean±standard deviation or median (interquartile range) values. The Shapiro-Wilk test was used to assess normality. Analysis of variance or Kruskal-Wallis test was used to compare differences in the means of continuous variables between the 3 groups with parametric and nonparametric data, respectively. The difference within 2 subsets was analyzed using the Bonferroni method with P=0.025. The chi-square test for trend analysis was used to compare the proportions of the 3 groups. Logistic regression analysis was performed to find risk factors for postoperative hypoPTH. The multivariate-adjusted model was constructed with the variables found to be significant (P<0.1) in univariate analysis. Statistical significance was defined as P<0.05 (2-tailed).
5. Ethical statement
This study was performed according to the Declaration of Helsinki. The need for informed consent was waived by the Institutional Review Board (IRB) of Seoul National University Hospital (IRB No. 2007-032-1139).
Results
1. Baseline characteristics
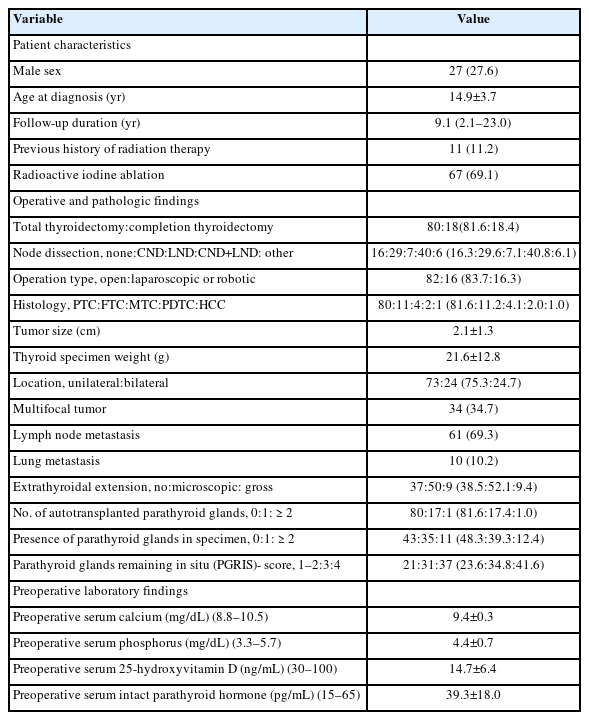
The demographic, clinical, and operative characteristics of 98 study participants (27 boys and 71 girls) are described in Table 1. The mean age at diagnosis of thyroid cancer was 14.9±3.7 years. The number of childhood cancer survivors who had received radiation therapy was 11 (11.2%). After surgery, 67 (69.1%) received RAIT for remnant ablation. For initial treatment, 80 (81.6%) underwent TT, while the remaining 18 (18.4%) received completion thyroidectomy after subtotal thyroidectomy or lobectomy. Sixty-nine patients (70.4%) underwent CND with or without LND. Eighty-two (83.7%) received open thyroidectomy, while others received laparoscopic or robotic surgery (n=16, 16.3%). The pathological diagnoses were 80 papillary thyroid cancers (81.6%), 11 follicular thyroid cancers (11.2%), 4 medullary thyroid cancers (4.1%), 2 poorly differentiated thyroid cancers (2.0%), and 1 Hürthle cell carcinoma (1.0%). The size of the tumor was 2.1±1.3 cm, and the weight of the thyroid specimen was 21.6±12.8 g. The rates of bilaterality, multifocality, lymph node metastasis, and lung metastasis were 24.7%, 34.7%, 69.3%, and 10.2%, respectively. Microscopic and gross ETE presented in 52.1% and 9.4% of cases, respectively. PTGs were autotransplanted in 18 patients (18.4%) during operation, and incidental parathyroidectomy was reported in 46 patients (51.7%), with a single PTG documented in 35 patients (39.3%) and 2 or more PTGs documented in 11 patients (12.4%). The numbers (%) of patients with PGRIS scores of 1–2, 3, and 4 points were 21 (23.6%), 31 (34.8%), and 37 (41.6%), respectively. The mean concentrations of calcium (9.4±0.3 mg/dL; reference range, 8.8–10.5 mg/dL), phosphorus (4.4±0.7 mg/dL; reference range, 3.3–5.7 mg/dL), and iPTH (39.3±18.0 pg/mL, reference range, 15–65 pg/mL) before the operation were within normal ranges. A low serum level of 25OHD was detected (14.7±6.4 ng/mL; reference range, 30–100 ng/mL), although it was only measured in 9 patients.
2. Postoperative hypoPTH and follow-up course
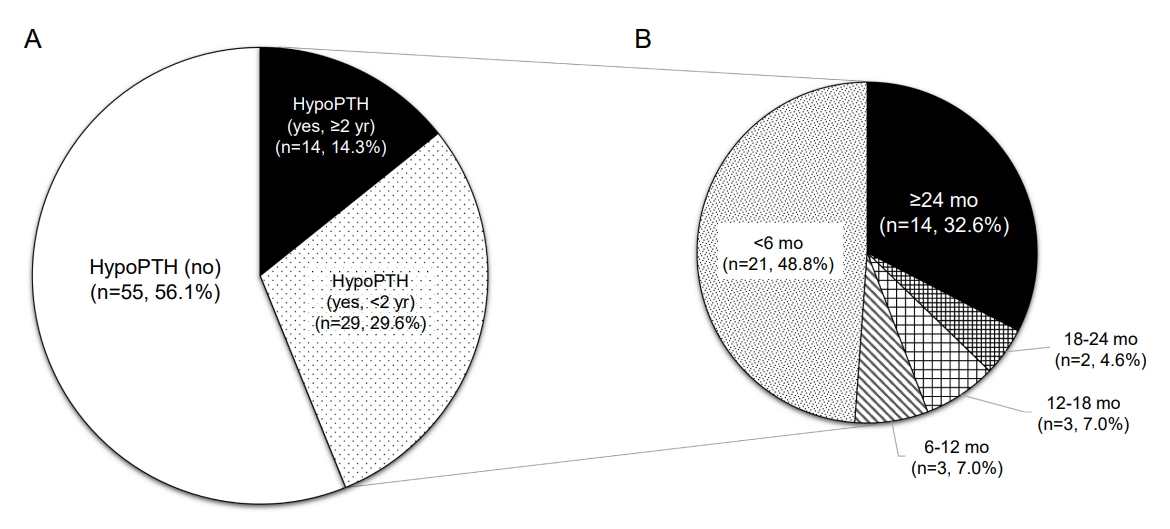
Forty-three patients (43.9%) were diagnosed with hypoPTH, which was defined as postoperative hypocalcemia requiring active vitamin D (Fig. 2A). During a median follow-up period of 9.1 years (range, 2.1–23.0 years), 29 patients discontinued active vitamin D medication; discontinuation was assessed at less than 6 months (n=21), 6–12 months (n=3), 12–18 months (n=3), and 18–24 months (n=2) (Fig. 2B). The remaining 14 patients on active vitamin D therapy continued the medication for more than 24 months after surgery.
3. Comparison according to duration of active vitamin D medication
The preoperative calcium level was significantly lower in the hypoPTH (yes, ≥2 years) group compared to the hypoPTH (no) group (9.2±0.3 mg/dL vs. 9.5±0.3 mg/dL, P<0.025) (Table 2). There were no significant differences in age at diagnosis, sex, pathologic diagnosis, neck dissection modality, multifocality, lymph node and lung metastasis, ETE, or PGRIS score among the 3 hypoPTH groups (Table 2).
4. Risk factors for postoperative hypoPTH
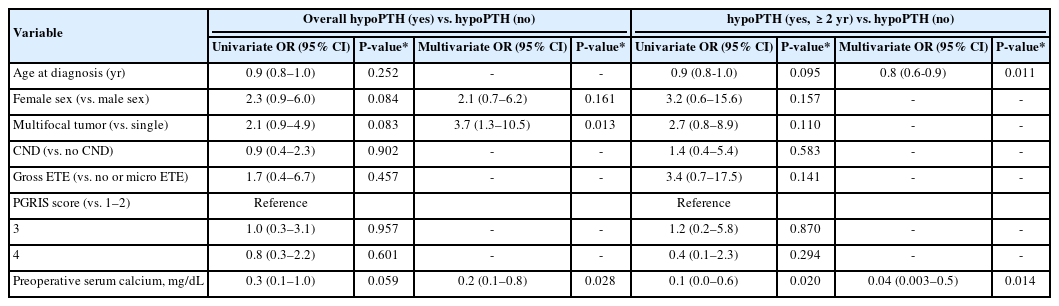
Table 3 includes the results of univariate and multivariate analyses for risk of overall hypPTH (yes) and hypoPTH (yes, ≥2 years) compared to hypoPTH (no). In multivariate-adjusted models including possible risk factors identified during univariate analysis, multifocality (odds ratio [OR], 3.7; 95% confidence interval [CI], 1.3–10.5; P=0.013) and preoperative calcium level (OR, 0.2; 95% CI, 0.1–0.8; P=0.028) were independent predictors of hypoPTH (yes). For hypoPTH (yes, ≥2 years), age (OR, 0.8; 95% CI, 0.6–0.9; P=0.011) and preoperative calcium level (OR, 0.04; 95% CI, 0.003–0.5; P=0.014) were significant predictors. No risk factors were identified during univariate analysis for hypoPTH (yes, <2 years) compared to hypoPTH (no) and hypoPTH (yes, ≥2 years).
Discussion
Among pediatric patients with thyroid cancer in a large tertiary center in Korea, postoperative hypoPTH occurred in 43.9% after TT. Among them, the proportion of patients who continued active vitamin D medication for more than 2 years was 32.6% (14.3% of the total). Tumor multifocality and preoperative calcium level were predictive for postoperative hypoPTH, and young age at diagnosis and low preoperative calcium level were risk factors for persistent hypoPTH requiring vitamin D for more than 2 years.
The frequency of overall postoperative hypoPTH (43.9%) in this study was higher than that in previously published reports. The frequencies of transient and permanent hypoPTH in pediatric thyroid cancer patients were reported to be 7.4%–33.1% and 0%–23.8% in previous studies [8-18]. The incidence rate differs depending on the indication or extent of surgery, lymph node dissection modality, area of study conduction, and follow-up duration [9-19,22,24] as well as definition of hypoPTH using different time points (6 months after operation [11,12,15,24], 1 year after operation [22] or at last follow-up [9]), biochemical values(serum calcium [15], ionized calcium [25], or PTH level [9,10]), or treatment requirements (active vitamin D and/or calcium [11,12,16,22,24] supplementation postoperatively). Since there have been variable definitions of postsurgical hypoPTH used and the study protocols differed among institutions, the incidence rate of hypoPTH differs significantly among studies, which makes it hard to compare rates between them.
In this study, tumor multifocality was identified as a risk factor for overall hypoPTH. The risk for postoperative hypoPTH increased when PTGs were removed inadvertently or devascularized by ligation of the blood supply during surgery. Considering the association between multifocal tumor and advanced stage at diagnosis [26,27], more extensive surgery adversely affected the blood supply of PTGs during operation [5,26,28], leading to increased risk of hypoPTH. One study [17] including 740 pediatric thyroid cancer patients in Belarus also demonstrated tumor multifocality as a significant risk factor for hypoPTH in association with tumor extension and degree of surgical aggressiveness. Previous studies also have identified other risk factors of extensive surgery, such as lymph node or lung metastasis and gross ETE [4,9,10] contributing to postoperative hypoPTH, although this trend was not demonstrated in our study.
Preoperative calcium level is a common risk factor for hypoPTH immediately after surgery and for persistent hypoPTH requiring active vitamin D therapy for more than 2 years. Lower level of preoperative calcium [25,29] and vitamin D deficiency [30-32] have been identified as risk factors for hypoPTH after thyroid surgery. Although we could not evaluate the relationship between preoperative calcium level and vitamin D deficiency in all patients, 8 of 9 who underwent 25OHD measurements were vitamin D-deficient in this study. Considering the importance of sufficient calcium and 25OHD levels preoperatively in previous studies [25,29-32] and the high prevalence of vitamin D deficiency in Korean adolescents [33], it is recommended to measure both calcium and 25OHD levels before thyroidectomy and to maintain a sufficient serum calcium concentration.
The risk of persistent hypoPTH requiring active vitamin D for more than 2 years increased when age at diagnosis was younger. Younger age is not only a significant risk factor predicting poor prognosis in pediatric thyroid cancer [34], but also a predictor of endocrine-specific complications such as recurrent laryngeal nerve injury and hypoPTH [9,35,36]. Reasons for the greater incidence of hypoPTH in children include anatomical difficulties with a narrow surgical field of view, emphasizing the need for a more delicate operation by a skillful surgeon [37].
The extent of lymph node dissection and the PGRIS score did not predict the occurrence of hypoPTH in this study. It remains controversial whether the lymph node dissection modality affects the occurrence of postoperative hypoPTH. Several studies [9,11,12] have reported that CND increases the risk of both transient and permanent hypoPTH. However, other studies showed no clear correlation [10,37] since the risk of PTG sacrifice was mitigated by extracareful operation of experienced surgeons to retain PTGs. A higher PGRIS score, indicating that more PTGs were preserved in situ, has been identified as a preventive factor for hypoPTH in both adult [22] and pediatric [38] studies. However, our study included only pediatric thyroid cancer cases, in contrast with previous studies including both benign and thyroid malignancies [22,38]. More aggressive exploration to investigate possible nodal metastasis during cancer surgery may adversely affect the blood supply compared to cases of benign thyroid disease [39], although PTGs are retained in situ. This may explain why a high PGRIS score was not a preventive factor for hypoPTH in this study.
This study was limited by its cross-sectional, retrospective design and small sample size. Second, the limited data on preoperative 25OHD concentrations represent an additional limitation. Third, the postoperative iPTH level, which is a useful predictor of postoperative hypoPTH [40], was not prospectively investigated in all patients.
In conclusion, postoperative hypoPTH occurred in two-fifth of patients after TT in pediatric thyroid cancer, with one-third of them (one-seventh of the total) requiring medication for more than 2 years. Young patients, those with multifocal tumors, and those with low preoperative calcium level should be monitored intensively for hypoPTH after thyroidectomy. Efforts to prevent hypocalcemia and ensure adequate calcium concentration before surgery are warranted.
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Funding
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data availability
The data that support the findings of this study can be provided by the corresponding author upon reasonable request.
Author contribution
Conceptualization: YAL, YJL, CHS; Data curation: YC; Formal analysis: YC, CHS, EJC; Methodology: YAL, YC, YJL, EJC; Visualization: YC; Writing - original draft: YC; Writing - review & editing: YAL, YJL, CHS, EJC