Temporal trends in the prevalence of metabolically healthy overweight and obesity in Korean youth: data from the Korea National Health and Nutrition Examination Survey 2011–2019
Article information
Abstract
Purpose
Metabolically healthy overweight/obesity (MHO) and metabolically unhealthy overweight/obesity (MUO) are distinct clinical phenotypes classified by the presence of cardiometabolic risk factors in an individual. In the present study, we investigated temporal trends in the prevalence of MHO in Korean adolescents using nationally representative data.
Methods
Data from the Korea National Health and Nutrition Examination Survey 2011–2019 were used in this study. A total of 5,667 adolescents (3,014 boys, 53.2%) aged 10–18 years was included in this study. MHO was defined as a body mass index ≥85th percentile for the corresponding age and sex and absence of any cardiometabolic risk factors.
Results
The prevalence of overweight/obesity showed an increasing trend from 18.8% (boys 17.3% and girls 20.6%) in 2011 to 23.7% (boys 24.0% and girls 23.5%) in 2019 (P for trend=0.045). The overall prevalence of MHO during 2011–2019 was 39.2%, which was higher in girls than in boys (boys 33.5%, girls 46.2%, P<0.001), and the change in prevalence of MHO from 2011 to 2019 (from 34.8% to 35.7%) was not significant. Among MUO, the most prevalent cardiometabolic risk factor was dysglycemia (48.8%), followed by elevated blood pressure (41.5%), low high-density lipoprotein cholesterol (35.0%), and high triglycerides (29.7%).
Conclusions
We observed a high prevalence of MHO in Korean youth with overweight/obesity. Although the prevalence of overweight/obesity increased, the prevalence of MHO was stable during 2011-2019. A risk-stratified approach based on metabolic health status can help reducing the medical and socioeconomic costs associated with obesity treatment.
Highlights
· Metabolically healthy overweight/obesity (MHO) is defined as the absence of cardiometabolic risk factors in an individual.
· The prevalence of MHO in Korean youth was stable during 2011-2019.
· The ultimate goal is returning overweight and obese youth to a metabolically healthy normal-weight.
Introduction
During the past four decades, the prevalence of overweight and obesity in children and adolescents has risen substantially across most countries [1]. Children who are overweight or obese have an increased risk of accompanying cardiometabolic risk factors (CMRFs), such as high blood pressure (BP), dyslipidemia, glucose intolerance, and/or insulin resistance [2]. Furthermore, overweight and obese status in childhood can persist in adulthood, which can lead to cardiovascular morbidity and reduced economic productivity in these individuals during adulthood [3,4].
Recently, overweight and obesity have been increasingly recognized as a heterogeneous condition wherein a subset is absent of any CMRFs, termed metabolically healthy overweight/obesity (MHO) [5]. Stratification of overweight and obese individuals based on metabolic health status can benefit the optimization of therapeutic and preventive approaches accordingly. Although some studies in adults have reported that individuals with MHO are at a higher risk of type 2 diabetes mellitus or cardiovascular diseases than healthy normal-weight adults [6,7], studies on long-term health outcomes of MHO in childhood are lacking. In addition, there has been no universally accepted criteria to identify individuals with MHO, and the absence of metabolic syndrome (MetS) and/or insulin resistance has been used to define MHO in most studies [5]. A consensus report in 2018 presented the definition of MHO in children, which includes high-density lipoprotein cholesterol (HDL-C), triglycerides (TG), BP, and glucose in the definition [8].
The MHO phenotype seems to be more frequent in children and adolescents than in adults. A previous Korean study based on the Korea National Health and Nutrition Examination Survey (KNHANES) IV (2007–2009) showed that the prevalence of MHO was between 36.8% (with no CMRF) and 68.8% (without insulin resistance) in Korean adolescents aged 10–19 years [9]. However, no study has investigated the temporal changes in MHO in children and adolescents. In the present study, we examined the recent nine-year trend of the prevalence of overweight/obesity according to metabolic health status in Korean youth, based on the KNHANES conducted from 2011 to 2019.
Materials and methods
1. Subjects
The present study was based on data obtained from the KNHANES between 2011 and 2019. KNHANES is an ongoing cross-sectional national survey started in 1998, that is composed of three component surveys: a health interview, health examination, and nutrition survey. This survey uses a stratified, multistage, clustered probability sampling method to select a nationally representative sample of noninstitutionalized citizens residing in Korea. All statistics of this survey were calculated using sample weights assigned to the participants. Detailed information about KNHANES has been published by the Korea Centers for Disease Control and Prevention [10]. Of the total 71,903 participants from the 2011–2019 surveys, 7,213 adolescents aged 10–18 years were included in our study. We excluded participants with missing anthropometric data (n=550), those who did not fast for at least eight hours prior to sampling (n=833), and those with missing values for any of the following measures: HDL-C, TG, BP, and glucose (n=1,438). After these exclusions, 5,667 participants (3,014 boys) were included in the present study. All survey participants provided informed consent. The Institutional Review Board of the Korea Centers for Disease Control and Prevention approved the use of these data.
2. Anthropometric and laboratory measurements
Height and weight were measured using standard methods while the participants wore light clothes without shoes or jewelry. Body mass index (BMI) was calculated as weight (kg) divided by height squared (m2). The z-scores for height, weight, and BMI were assigned on the basis of the 2017 Korean National Growth Charts [11]. Waist circumference was measured from the midpoint between the lower end of the last rib cage and the upper rim of the iliac crest and recorded to the nearest 0.1 cm. Systolic and diastolic BPs were obtained using a standard mercury sphygmomanometer (Baumanometer Desk Model 3020, WA Baum, Co., Copiague, NY, USA) with participants in a seated position. Blood samples were obtained from the participants by venipuncture in the morning after an overnight fast. The serum levels of total cholesterol, HDL-C, TG, and glucose were measured enzymatically using a Hitachi Automatic Analyzer 7600 (Hitachi, Tokyo, Japan) in the 2011–2012 surveys, a Hitachi Automatic Analyzer 7600-210 (Hitachi) in the 2013–2018 surveys, and a Labospect008AS (Hitachi) in the 2019 survey. Glycated hemoglobin (HbA1c) was measured by high-performance liquid chromatography using an HLC-723G7 (Tosoh, Tokyo, Japan), which is certified by the National Glycohemoglobin Standardization Program. Self-reported questionnaires were used to assess daily energy intake (kcal/day) using a single 24-hour dietary recall method.
3. Definitions of MHO and MUO
We defined overweight (85th to <95th BMI percentile) and obesity (≥95th BMI percentile) according to BMI percentiles [12]. MHO was defined as overweight or obese individuals who satisfied all of the following criteria: HDL-C >40 mg/dL; TG ≤150 mg/dL; systolic and diastolic BP ≤90th percentile for age, sex, and height; and normoglycemia [8]. Normoglycemia was defined as fasting glucose <100 mg/dL and HbA1c <5.7% [13]. Individuals without any of these findings were classified as metabolically unhealthy overweight/obesity (MUO) [8].
4. Statistical analysis
Statistical analyses were performed using Stata 16.1 software (StataCorp LP, College Station, TX, USA). All analyses were performed using sampling weights to report estimates representative of the Korean population. All continuous variables were expressed as weighted means with standard errors and categorical variables as numbers and weighted percentages of participants. Logarithmic conversions were performed for total cholesterol, HDL-C, and TG to approximate a normal distribution, as these variables were not normally distributed. Student t-test was used to compare the mean values of the continuous variables, and the chi-square test was used to compare categorical variables. Linear or logistic regression analyses were used to assess temporal trends of continuous or categorical variables. A P-value <0.05 was considered statistically significant.
Results
1. Demographic characteristics
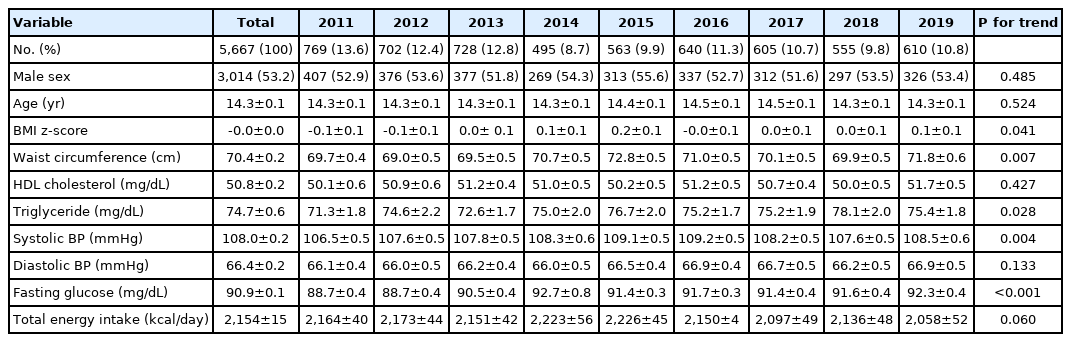
Table 1 shows the characteristics of 5,667 participants (3,014 boys, 53.2%) aged 10–18 years during the survey period of 2011–2019. The BMI z-scores significantly increased from –0.1 in 2011 to +0.1 in 2019 (P for trend=0.041). There was a significant increasing trend of waist circumference (from 69.7 to 71.8 cm), TG (from 71.3 to 75.4 mg/dL), systolic BP (from 106.5 to 108.5 mmHg), and fasting glucose level (from 88.7 to 92.3 mg/dL) from 2011 to 2019 (P for trend <0.05 for all). The total energy intake did not change significantly across the survey years.
2. Trends in the prevalence of overweight/obesity according to metabolic health
Table 2 shows the prevalence of overweight/obesity, MHO, and MUO and metabolic components from 2011 to 2019. The prevalence of overweight/obesity increased from 18.8% in 2011 to 23.7% in 2019 (P for trend=0.045) (Fig. 1A). An increasing tendency was noted only in boys (from 17.3% to 24.0%; P for trend=0.056), without significant change in girls (from 20.6% to 23.5%) (Fig. 1B). Among overweight and obese adolescents, the change in MHO prevalence (from 34.8% in 2011 to 35.7% in 2019) was not significant across the survey years (Fig. 2A). When stratified by sex, the overall prevalence of MHO was significantly higher in girls (46.2%) than in boys (33.5%) during 2011–2019 (P<0.001). The temporal change in MHO prevalence was not significant in either boys (from 26.8% to 29.4%) or girls (from 42.4% to 57.5%) (Fig. 2B).
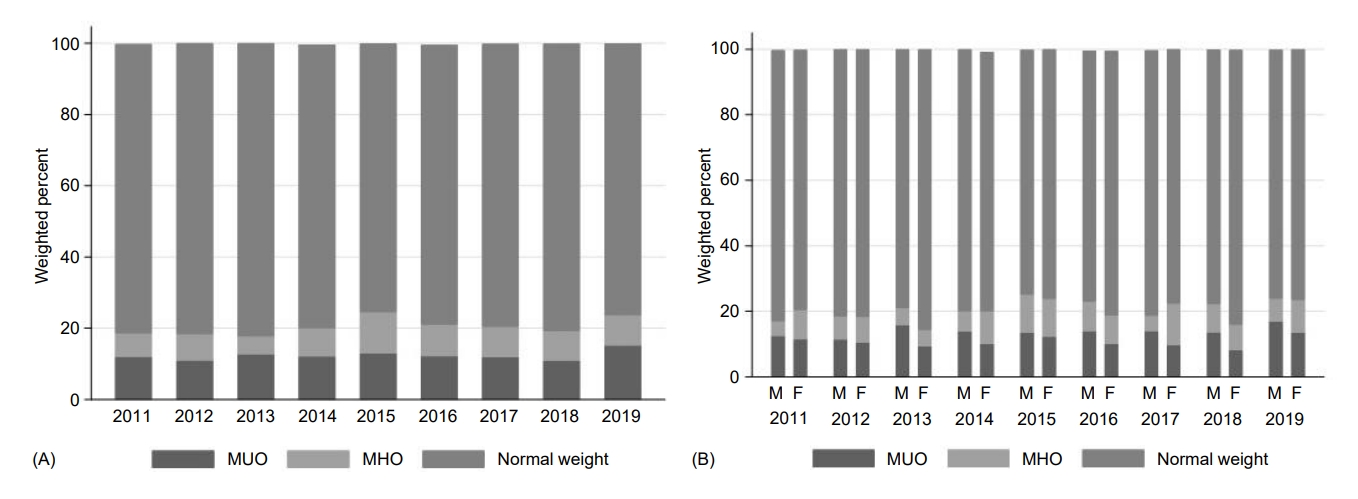
Prevalence of total (A) and sex-specific MUO, MHO, and normal weight (B) youth in Korea by year. MUO, metabolically unhealthy overweight/obesity; MHO, metabolically healthy overweight/obesity; M, male; F, female.
3. Trends in the prevalence of CMRFs in MUO
Among adolescents with MUO, the most prevalent CMRF was high fasting glucose level (48.8%), followed by high BP (41.5%), low HDL-C (35.0%), and high TG (29.7%) during 2011–2019. The temporal trends in the prevalence of each CMRF were not significant across the study period, showing no significant changes in sex-stratified analysis. In boys, dysglycemia including high fasting glucose or HbA1c (45.8%) was the most prevalent CMRF, followed by high BP (41.1%), low HDL-C (38.9%), and high TG (27.6%). In girls, the prevalence of CMRFs was highest in dysglycemia (53.2%), followed by high BP (42.0%), high TG (33.1%), and low HDL-C (29.2%) during the study period (Table 2).
Discussion
Based on large representative nationwide data in Korea, the prevalence of MHO in Korean adolescents showed a stationary trend from 2011 to 2019, although the prevalence of overweight/obesity significantly increased during the same period. Overall, the prevalence of MHO was 39.2% for overweight/obese, was higher in girls (46.2%) than in boys (33.5%), and did not show significant temporal change.
The observed increase in the prevalence of overweight/obesity among Korean adolescents during 2011–2019 is consistent with the global increasing trend of overweight/obesity in children, as well as in adults. [14]. Recent Korean studies using nationally representative data also reported an increase in the prevalence of overweight/obesity or BMI among children and adolescents, especially among high school students [15,16]. Although the change in total energy intake was not significant in the present study, changes in the food environment such as a high intake of fast food and soft drinks, decreased consumption of fruit and vegetables, and frequent skipping of breakfast, along with sedentary behaviors in Korean adolescents might be potential drivers [17]. Childhood overweight/obesity is associated with an increased prevalence of CMRFs, such as impaired glucose metabolism, hypertension, and dyslipidemia, in childhood or later in life [18,19]. In addition, the obesity pandemic in childhood might generate an excessive health burden for society [14]. However, the individual risk of developing obesity-related comorbidity varies greatly, and a subgroup of overweight and obese individuals did not exhibit cardiometabolic abnormalities, leading to the concept of MHO.
The MHO phenotype was described in the 1980s; however, no universal definition had been established, either in adults or children [20,21]. According to a systematic review, the prevalence of MHO in adults ranged from 6% to 75% and was higher in younger age, female, and Asian populations [22]. In children and adolescents, the reported prevalence of MHO varies from 3% to 80%, depending on the criterion used [21]. According to a recent consensus-based definition of MHO, defined as free of any CMRF [8], the MHO prevalence in Korean adolescents (39.2% in 2011–2019) was comparable with the reported MHO prevalence in European adolescents (41.1% in 2014–2019) [23]. Conversely, the present findings showed a higher prevalence of MHO than those found in Canadian (25% in 2010s) [24], and United States (21.4% of overweight and 5.7% of obese in NHANES 2007–2016) [25] adolescent-based studies that used the MetS criteria for the MHO phenotype. However, caution should be taken when comparing the data with other studies due to the differences in age group, survey years, and combination and cutoffs for CMRFs to define MHO across the studies.
Temporal trends in the prevalence of MHO have not been reported in previous studies. In the present study, the changes in MHO prevalence were not significant over the past nine years (from 34.8% to 35.7%), with similar prevalence (36.8%) reported in a previous Korean study using KNHANES IV (2007–2009) [9]. The higher prevalence of MHO in girls in the present study was in accordance with previous findings in obese children and adolescents [26-28]. Although the reasons for the sex differences in MHO prevalence are not well understood, hormonal differences, lifestyle factors such as physical activity and sedentary behaviors, and/or body fat distribution have been postulated to be possible explanations for the observed sex differences [26,29]. In terms of efficient care of overweight/obesity in childhood, the investigation of MHO has the potential to benefit the delivery of optimal health services for obesity management. In addition, given the possible protective effects of MHO on disease risk compared to MUO, it would be valuable to investigate and identify the factors that are associated with MHO status in youth to prevent obese individuals from developing metabolic abnormalities [30-32]. Regarding the determinants of metabolic health status, favorable lifestyle factors, such as healthy diet pattern and being less sedentary and more physically active, have been reported to be positively associated with MHO in childhood [5,9,33-35].
There is an ongoing debate as to whether MHO represents true health among obese individuals. Prospective studies tracking the development of cardiometabolic disease and mortality in MHO have presented conflicting results, which might be partly due to the heterogeneity of MHO definitions and the transient nature of MHO status [36]. The Bogalusa Heart Study showed that an MHO phenotype that onset in childhood was more likely to continue into adulthood [37].Conversely, the San Antonio Heart Study revealed that 47.6% of MHO adults transitioned to MUO during a median 7.8 years of follow-up [38]. In addition, 44% of European MHO adolescents became MUO over a 13-month longitudinal analysis [23]. Although long-term risks for diabetes, cardiovascular diseases, and mortality were lower among the MHO compared with the MUO, MHO adults showed increased risk of diabetes and incident cardiovascular disease relative to the metabolically healthy normal-weight individuals, especially among those who worsened to MUO [6,39]. Therefore, the goal of this risk stratification approach should be improving cardiometabolic profiles in overweight and obese youth and ultimately returning them to a metabolically healthy normal-weight.
This study has some limitations. First, there is the possibility of selection bias associated with declining participation rates among overweight and obese individuals. Second, the effect of pubertal stage on the MHO phenotype could not be evaluated because of the lack of information in the KNHANES database. Finally, although we examined temporal trends by combining data from consecutive national surveys, this was a cross-sectional design; hence, we could not track individual changes in the MHO phenotype and its associated lifestyle factors. Nevertheless, to the best of our knowledge, this is the first study to show the trend of MHO status in childhood using a large nationally representative database.
In conclusion, this study provided recent data on the increasing prevalence of overweight/obesity but the stable prevalence of MHO in Korean adolescents. Further longitudinal studies exploring factors associated with metabolic health and long-term consequences of MHO in overweight and obese children are warranted.
Ethical statement
Informed consent was obtained from all individuals who participated in the KNHANES. The study protocol was approved by the Institutional Review Board (IRB) of Seoul National University Bundang Hospital (IRB No. X-1907-555-906). All procedures were performed in accordance with the Declaration of Helsinki.
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Funding
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data availability
The data that support the findings of this study are available via https://knhanes.kdca.go.kr/knhanes/sub03/sub03_02_05. do.
Author contribution
Conceptualization: HYK, JHK; Data curation: HYK, JHK; Formal analysis: JHK; Methodology: JHK; Writing - original draft: HYK; Writing - review & editing: HYK, JHK