Clinical practice guidelines for optimizing bone health in Korean children and adolescents
Article information
Abstract
The Committee on Pediatric Bone Health of the Korean Society of Pediatric Endocrinology has newly developed evidence-based clinical practice guidelines for optimizing bone health in Korean children and adolescents. These guidelines present recommendations based on the Grading of Recommendations, which includes the quality of evidence. In the absence of sufficient evidence, conclusions were based on expert opinion. These guidelines include processes of bone acquisition, definition, and evaluation of low bone mineral density (BMD), causes of osteoporosis, methods for optimizing bone health, and pharmacological treatments for enhancing BMD in children and adolescents. While these guidelines provide current evidence-based recommendations, further research is required to strengthen these guidelines.
Highlights
The Committee on Pediatric Bone Health of the Korean Society of Pediatric Endocrinology has newly developed evidence-based clinical practice guidelines for optimizing bone health in Korean children and adolescents. These guidelines include processes of bone acquisition, definition, and evaluation of low bone mineral density (BMD), causes of osteoporosis, methods for optimizing bone health, and pharmacological treatments for enhancing BMD in children and adolescents.
Introduction
Osteoporosis was previously thought to be a disease exclusive to older adults. However, it is now increasingly recognized in younger adults due, in part, to the longer survival of chronically ill children. Children with osteoporosis are at a high risk of skeletal morbidity not only during youth, but also in adult life [1]. Bone mass attained in early life is thought to be the most important modifiable determinant of lifelong skeletal health [2]. In this context, the Committee on Pediatric Bone Health of the Korean Society of Pediatric Endocrinology recently developed evidence-based clinical practice guidelines for optimizing bone health in Korean children and adolescents. Recommendations were determined according to the quality of evidence graded by the steering committee on quality improvement and management in the American Academy of Pediatrics [3].
This guideline comprises the physiological processes of bone acquisition, definition, and evaluation of low bone mineral density (BMD), causes of osteoporosis, conservative management for optimizing bone health, and pharmacological treatments for enhancing BMD in children and adolescents.
Bone acquisition and peak bone mass
Bone is a living structure comprising a matrix of collagen, hydroxyapatite crystals, and noncollagenous proteins. The matrix becomes mineralized with deposits of calcium and phosphate, which confer strength to the structure [1].
Bone mineral deposition begins in utero, and bone mineral content increases 40-fold from birth to adulthood. BMD of the lumbar spine (BMDLS) showed the greatest increase from the age of 11 to 13 years in girls and from 12 to 14 years in boys. BMD of the total body less head (BMDTBLH) shows a relatively modest increase from the age of 10 to 15 years in girls and from 10 to 18 years in boys [4,5]. In Korea, peak bone mass is achieved at approximately 19 and 21 years of age in girls and boys, respectively [6,7].
There are numerous factors that affect bone mineral deposition and bone mass formation. These are largely divided into 2 groups: nonmodifiable factors including genetics, sex, and ethnicity and modifiable factors including nutrition, exercise, lifestyle, body weight and composition, and hormonal status [8].
BMD measurement
BMD in children and adolescents is most commonly assessed with dual-energy x-ray absorptiometry (DXA) because it is less time-consuming and more precise and widely available, safer, and less costly than other options [9]. The posteroanterior lumbar spine and total body less head are the preferred skeletal sites for BMD measurement in most pediatric patients, while other sites are recommended only for specific cases [9]. BMDLS measurement is a feasible and reproducible method, especially for infants and young children aged 0–5 years. BMDTBLH measurement can be used for children aged 3 years and older [9]. The standard methods of BMD evaluation in children and adolescents are performed using BMD z-score calculations according to comparisons with reference data in children of each age, sex, pubertal stage, and ethnicity [6,7,9]. In children with short stature or growth delay, BMDLS should be adjusted with bone mineral apparent density or height z-score and BMDTBLH with the height z-score [9].
Two types of bone densitometers (GE Lunar Prodigy Advance bone densitometer, Absolute Medical Inc, Stony Point, NY, USA and Hologic QDR densitometer, Hologic Inc, Bedford, MA, USA) were used. Normal BMD reference values for Koreans were compared to each densitometry measurement [6,7].
Definition of low BMD and osteoporosis
Low BMD is the preferred term for DXA reports in children when the BMD z-score is less than or equal to -2.0 standard deviation (SD) [9]. Osteoporosis in children is defined as the presence of ≥1 vertebral compression fracture in the absence of local disease or high-energy trauma or the presence of both a clinically significant fracture history and a BMD z-score of -2.0 or lower. A clinically significant fracture history includes at least one of the following: (1) ≥2 long bone fractures by the age of 10 years and (2) ≥3 long bone fractures at any age up to 19 years [9].
Conditions associated with low BMD
There are primary and secondary causes of reduced bone mass and increased fracture risk (Table 1). Primary osteoporosis occurs due to intrinsic skeletal defects originating from genetic or idiopathic causes, while secondary osteoporosis results from chronic systemic illnesses in children due to either the effects of the disease process on the skeleton or from treatment [8,10,11].
1. Primary osteoporosis
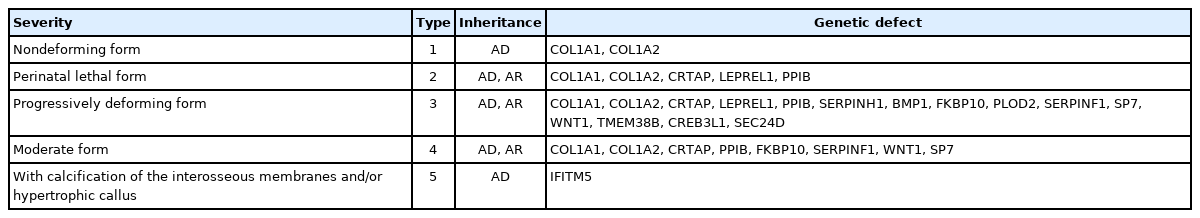
1) Osteogenesis imperfecta
Osteogenesis imperfecta is a rare genetic disorder with an incidence of approximately 1/25,000. Clinical manifestations include low bone mass, frequent fractures, bone deformity, short stature, wormian bones, blue sclerae, hypermobility of joints, dentinogenesis imperfecta, and deafness. There are 5 types of osteogenesis imperfecta according to clinical severity. Type 1 is the least severe and nondeforming; type 2 is lethal in the perinatal period because of respiratory insufficiency due to multiple rib fractures; type 3 is associated with limb deformities and is progressively deforming; type 4 has an intermediate phenotype between types 1 and 3; and type 5 has a phenotype similar to that of type 4 but is associated with calcification of interosseous membranes and hypertrophic callus formation at healing fracture sites. The most common mutations in all types are found in COL1A1 and COL1A2, but novel mutations in other genes have been found (Table 2) [10].
2) Idiopathic juvenile osteoporosis
Idiopathic juvenile osteoporosis is a disease of unknown etiology that is considered genetically heterogeneous in origin. The clinical manifestations include bone pain and fractures with minimal trauma in girls or boys aged 8–12 years. Symptom severity tends to abate spontaneously with variable residual manifestations [10,12]. Differential diagnosis of osteogenesis imperfecta and idiopathic juvenile osteoporosis is shown in Table 3.
3) Osteoporosis-pseudoglioma syndrome
Osteoporosis-pseudoglioma syndrome is a congenital or infantile-onset disease. Its clinical manifestations include severe visual impairment, bone fragility, ligamentous laxity, hypotonia, and cognitive impairment. It is transmitted as an autosomal recessive trait and is associated with several types of mutations in LRP5 [10].
2. Secondary osteoporosis
1) Endocrine disorders
Hypogonadism, growth hormone (GH) deficiency, hyperthy roidism or hypothyroidism, hyperparathyroidism, diabetes mellitus (DM), and Cushing syndrome are associated with low bone mass [8,10,11]. Hypogonadism is associated with decreases in cortical width, trabecular number, and osteoid and mineralization activity because of the decreased lifespan of osteoblasts and osteocytes but increased lifespan of osteoclasts, histomorphometrically [13] GH deficiency is associated with decreases in osteoblast differentiation and proliferation, osteoblast synthesis of insulin-like growth factor-I (IGF-I), insulin-like growth factor binding protein-3, osteocalcin, bone-specific alkaline phosphatase, procollagen type I, and osteoprotegerin [14,15].
Thyroid disorders also lead to secondary osteoporosis in children. In hyperthyroidism, rates of cartilage maturation, bone turnover, and osteoclastogenic effects increase [16]. In hypothyroidism, cartilage maturation and endochondral bone formation decrease. Additionally, thyroid hormone deficiency in early life delays the development of epiphyseal centers of ossification [16]. In hyperparathyroidism, excess parathyroid hormone induces the production of receptor activator for nuclear factor κB ligand (RANKL) and inhibits the production of osteoprotegerin, increasing osteoclastogenesis and bone resorption [11].
In DM, hyperglycemia is related to accumulation of glycated proteins, which impairs differentiation of mesenchymal stem cells into chondrocytes and osteoblasts with decreased synthesis of osteocalcin [17]. Finally, in Cushing syndrome, glucocorticoid excess is directly associated with inhibition of osteoblastogenesis and promotion of osteoblast and osteocyte apoptosis. Further, the excess glucocorticoid indirectly impairs bone accretion by inhibiting secretion of IGF-I and intestinal calcium absorption [18-20].
2) Neuromuscular disorders
Children with cerebral palsy, Duchenne muscular dystrophy, and spinal muscular atrophy cannot bear weight in the same way as children without these neuromuscular disorders, resulting in decreased bone formation with continued bone resorption and development of lower bone mass [21-24].
3) Connective tissue disorders
In children with systemic lupus erythematosus, juvenile idiopathic arthritis, and juvenile dermatomyositis, bone mass is decreased due to chronic inflammation, production of proosteoclastic cytokines, and glucocorticoid therapy [25,26].
4) Gastrointestinal disorders
Nutritional deficiency, secondary hyperparathyroidism, chronic inflammatory state, and glucocorticoid therapy can decrease bone formation and increase bone resorption in children with chronic inflammatory bowel or celiac disease [27-29].
5) Hemato-oncologic disorders
Cytokine-induced osteoclastogenesis, radiation- or chemotherapeutic agent-induced bone injury, decreased physical activity, and nutritional deficiency result in diminished bone mass in children with leukemia or cancer [30,31].
6) Renal disorders
Nutritional deficiency or immunosuppressive therapy can result in bone loss in children with chronic renal failure and kidney transplantation [32,33].
7) Nutritional deprivation
Anorexia nervosa and female athlete triad are examples of both nutrition- and hormone-induced bone loss that can lead to early-onset osteoporosis [8].
8) Medications
Numerous medications such as glucocorticoids, anticonvul sants, chemotherapeutic agents, immunosuppressive agents, heparin, proton pump inhibitors, and selective serotonin reuptake inhibitors can increase the risk of low bone mass [8]. In children, studies have reported that use of oral glucocorticoids for more than 3 months reduced bone density and increased the risk of spinal compression fractures. Therefore, it is recommended that glucocorticoids be used in low doses every other day rather than every day, while maintaining calcium and vitamin D concentrations in the normal range. As short-term administration or inhaled formulations of corticosteroid can also affect bone density [1], it is difficult to recommend safe doses and durations [19].
Evaluation in patient with low BMD or increased risk of fracture
In pediatric patients with primary bone disease or those at risk of secondary bone disease, DXA should be performed when the patient might benefit from interventions to decrease the risk of clinically significant fracture. If follow-up DXA is indicated, the minimum interval between scans should be 6–12 months [9]. The evaluation processes according to risk of osteoporosis are shown in Fig. 1.
Conservative management for optimizing bone health
Modifiable determinants of bone mass include nutrition, exercise, lifestyle, body weight, lean body mass, and hormonal status. These conditions should be adequately maintained to optimize bone health [8].
1. Nutrition
Many studies have reported that calcium and vitamin D supplementation is helpful in increasing bone mass and BMD in children. These nutritional elements should be supplemented using food rather than medicine in healthy children [34-37]. Many types of foods are good dietary sources of calcium and vitamin D. Human milk or infant formula is the primary source of calcium for healthy infants in the first year, and milk and other dairy products are the major sources of calcium after the first year of life. Other dietary sources include green leafy vegetables, legumes, nuts, cereal, and fruit juices. The bioavailability of calcium from vegetables is generally high but is reduced by binding with oxalate in spinach, collard greens, and beans and with phytate in whole bran cereals. Natural dietary sources of vitamin D include cod liver oil, salmon, sardines, tuna, and fortified foods. Exposure to ultraviolet B radiation from sunlight is the major source of vitamin D production [8]. The recommended daily allowance and upper limit of calcium and vitamin D are shown in Table 4 [8,36-39]. If calcium or vitamin D deficiency causes low BMD, it should be administered at a therapeutic dosage (Table 5) [8,40]. Several types of vitamin D prescriptions are available including ergocalciferol (vitamin D2), cholecalciferol (vitamin D3), alphacalcidol (1α-hydroxyvitamin D3), and calcitriol (1,25-dihydroxyvitamin D3). Vitamin D3, an active form of vitamin D, is frequently used in children with vitamin D deficiencies. Alphacalcidol or calcitriol is used in children with renal diseases [8].
Ingestion of adequate amounts of calories, proteins, and vegetables or fruits containing high vitamin C, vitamin K, potassium, magnesium, copper, and manganese contents is also needed to maintain bone health in children [41]. The prescription forms of calcium are calcium carbonate (40% elemental calcium), calcium citrate (21% elemental calcium), calcium lactate (13% elemental calcium), and calcium glutamate (9% elemental calcium). Calcium carbonate should be taken with meals to promote absorption, but calcium citrate can be taken on an empty stomach. The adverse effects of consuming excessive calcium are dyspepsia, constipation, urinary stones, and hypercalcemia, especially when taken with vitamin D [8].
2. Exercise
Mechanical forces applied to the skeleton increase bone formation, and weight-bearing exercises improve bone mineral deposition [42-45]. High-impact and low-frequency exercises such as jumping, skipping, and hopping for 10 minutes 3 times per week increases the BMD of the femoral neck in healthy children [46]. Weight-bearing exercises such as walking, jogging, jumping, and dancing are better than non-weight-bearing exercises such as swimming or bicycle riding [45,46]. However, excessive high-impact and prolonged exercises can increase fracture risk; therefore, children who want to participate in these sports should cross-train with low-impact activities [47].
3. Lifestyle
Ingestion of fast food, alcohol, caffeine, and smoking can promote bone loss and should be avoided [41]. Sedentary activities, such as prolonged TV watching, internet gaming, and cellular phone use, also have negative effects on bone mass [8,46].
4. Body weight and composition
Body mass index and lean body mass are directly correlated with BMD in most healthy adolescents; conversely, increased adiposity can be related with increased fracture risk. This implies the importance of maintaining healthy body composition during childhood and adolescence [8,48].
5. Hormonal status
Estrogen, testosterone, GH, and IGF-I c an promote bone formation, but excess glucocorticoid increases bone resorption [8,10,11]. Therefore, bone mineral depositions in children with endocrine disorders, such as hypogonadism, Turner syndrome, GH deficiency, and Cushing syndrome, should be carefully investigated and adequately maintained.
Pharmacological treatment for BMD enhancement
1. Bisphosphonate
1) Action mechanism and forms of bisphosphonate
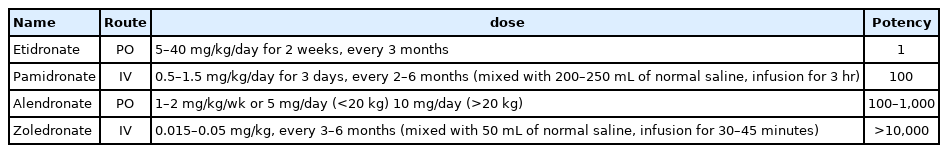
Bi sphosphonate s i n h ibit oste o cl a st - me d i ate d b one resorption and prevent osteoblast or osteocyte apoptosis. These medications have been used in children with osteogenesis imperfecta, diseases treated with corticosteroids, cerebral palsy, and connective tissue diseases. Bisphosphonates have been reported to increase BMD and decrease pain and fracture in children with these diseases. However, their use in children remains controversial because of long half-lives and potential adverse effects [49]. The various prescription forms of bisphosphonates are shown in Table 6.
2) Indication for bisphosphonate treatment
(1) Primary osteoporosis
Bisphosphonates are commonly prescribed for individuals with osteogenesis imperfecta. Many reports have demonstrated that oral or intravenous bisphosphonates increase BMD in children and adults with this condition [50,51]. Most patients with idiopathic juvenile osteoporosis tend to remit spontaneously, but if there is a possibility of permanent deformity after vertebral or long bone fracture, bisphosphonate treatment should be considered [52].
(2) Secondary osteoporosis
Bisphosphonates should be used in children with recurrent extremity fractures, symptomatic vertebral collapse, and severe bone pain caused by other clinical conditions but should not be used to treat asymptomatic reduction in bone mass in children [53]. In addition, sufficient nutrition including calcium/vitamin D, adequate body weight, physical activity within the possible range, and supplementation in cases of hormone deficiencies should be prioritized over bisphosphonate treatment [54]. Bisphosphonates have been reported to be effective for pediatric patients presenting with secondary osteoporosis with cerebral palsy, juvenile idiopathic arthritis, renal transplantation, renal disease with chronic steroid therapy, Crohn disease, Duchenne muscular dystrophy, leukemia or lymphoma with chemotherapy, or chronic graft-versus-host disease [55-64].
3) Practical bisphosphonate therapy
Although guideline consensus for bisphosphonate forms, dosage, treatment duration, and interval of administration is needed in children, pamidronate is often used in young children, followed by switching to zoledronate in older patients with moderate-to-severe disease [54]. Pamidronate doses vary from 9 to 12 mg/kg/yr in 4 to 6 divided doses, and zoledronate is initiated at 0.1 mg/kg/yr in 2 divided doses. The starting doses for pamidronate and zoledronate are usually recommended to be reduced to 0.5 mg/kg and 0.0125 or 0.025 mg/kg respectively, to minimize acute-phase reactions and hypocalcemia. Maintenance therapy at reduced doses is determined by a combination of fracture history, bone pain, BMD, and growth. The maintenance dosages for pamidronate and zoledronate are 3.0 and 0.025 mg/kg/yr, respectively, administered in 2 divided doses [54]. The typical treatment approaches for children with primary and secondary osteoporosis are shown in Fig. 2.
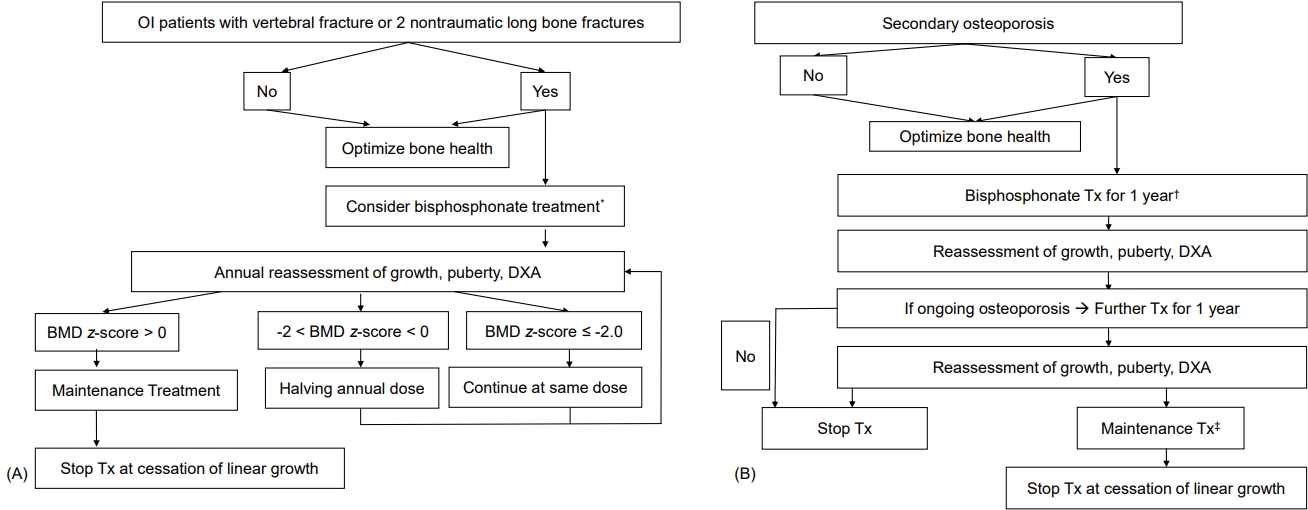
(A) Bisphosphonate treatment in patients with osteogenesis imperfecta (OI). (B) Bisphosphonate treatment in patients with secondary osteoporosis. DXA, dualenergy x-ray absorptiometry; BMD, bone mineral density; Tx, treatment. *Pamidronate 9 mg/kg/yr, 4–6 divided doses or zoledronate 0.1 mg/kg/yr, 2 divided doses. †Initial Tx of bisphosphonate, pamidronate 9 mg/kg/year, 4-6 divided doses or zoledronate 0.1 mg/kg/year, 2 divided doses. ‡Maintenance Tx, pamidronate 3 mg/kg/yr in 2 divided doses or zoledronate 0.025 mg/kg annually. Modified from Simm et al. J Paediatr Child Health 2018;54:223-33, with permission of Paediatrics and Child Health Division (The Royal Australasian College of Physicians) [54].
Contraindications for bisphosphonate therapy include pregnancy, severe renal disease, and active rickets. Reduced renal function is a relative contraindication because approximately 40%–60% of the dose is distributed to the bone; the remainder is excreted unaltered in the urine, with no substantial metabolism [65,66]. The acute adverse effects of bisphosphonate treatment in pediatric patients are flu-like symptoms and hypocalcemia, with possible long-term effects of delayed bone healing, osteopetrosis, and gastroesophageal reflux. The potential side effects are atypical femoral fractures, osteonecrosis of the jaw, orbital inflammation, growth impairment, and teratogenicity [65,66]. Therefore, clinical, biochemical, and imaging assessments must be performed [49,54]. General clinical examination with growth and nutritional evaluation, dental and eye examination, complete blood cell count, electrolytes, alkaline phosphatase, bone turnover markers, liver function test, renal function test, serum vitamin D and parathyroid hormone levels, urine analysis, urinary calcium excretion, radiography of the wrist and knee, and BMD should be included in the assessments [49,54].
2. New therapeutic agents
Several new drugs, such as teriparatide (recombinant human parathyroid hormone), strontium ranelate, and denosumab (monoclonal anti-RANKL antibody), increase bone formation and decrease bone resorption. However, reports on the effect and safety of these medications in children are insufficient [10,11,67].
Conclusion
Bone mineral deposition shows the highest increase during the pubertal period in girls and boys and can be a major determinant of BMD in later life. Therefore, modifiable determinants of bone mass, including nutrition, exercise, lifestyle, body weight, lean body mass, and hormonal status should be adequately maintained to optimize bone health in children and adolescents.
The definition of osteoporosis in children is different from that in adults. In pediatric patients with primary or secondary osteoporosis, underlying conditions should be treated first, and then conservative and pharmacologic therapies should be considered.
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Funding
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author contribution
Conceptualization: KSS; Data curation: AK, JHY, WKC; Formal analysis: JHK, JSL; Methodology: HN; Visualization: SYC; Writing - original draft: YA; Writing - review & editing: KSS
Acknowledgements
The Authors greatly appreciate the help from Pf. Choong Ho Shin (Past President of The Korean Society of Pediatric Endocrinology) and Pf. Ho Yeon Chung (Past Chairman of The Korean Society for Bone and Mineral Research) in organizing the committee for these guidelines. Further, Pf. Deog Yoon Kim (Past Chairman of The Korean Society for Bone and Mineral Research), Pf. Yumie Rhee (Secretary General, The Korean Society for Bone and Mineral Research), Pf. Seong Bin Hong (Director, Committee of Legislation and Ethics, The Korean Society for Bone and Mineral Research), Hye Ran Yang (Director of the External Cooperation Committee, The Korean Society of Pediatric Gastroenterology, Hepatology, and Nutrition), Ara Ko (The Korean Child Neurology Society), Joo Hoon Lee (Director of the Education and Training Committee, Korean Society of Pediatric Nephrology), Jun Ah Lee (Director of the Committee of Insurance, The Korean Society of Pediatric Hematology- Oncology) provided significant counsel for these guidelines.
Also, we would like to thank Eworldediting (www.eworldediting.com) & Editage (www.editage.co.kr)for English language editing.