Ambient air pollution and endocrinologic disorders in childhood
Article information
Abstract
Ambient air pollution has been proposed as an important environmental risk factor that increases global mortality and morbidity. Over the past decade, several human and animal studies have reported an association between exposure to air pollution and altered metabolic and endocrine systems in children. However, the results for these studies were mixed and inconclusive and did not demonstrate causality because different outcomes were observed due to different study designs, exposure periods, and methodologies for exposure measurements. Current proposed mechanisms include altered immune response, oxidative stress, neuroinflammation, inadequate placental development, and epigenetic modulation. In this review, we summarized the results of previous pediatric studies that reported effects of prenatal and postnatal air pollution exposure on childhood type 1 diabetes mellitus, obesity, insulin resistance, thyroid dysfunction, and timing of pubertal onset, along with underlying related mechanisms.
Highlights
There is growing evidence for a relationship between ambient air pollution and altered metabolic and endocrine systems in children. Further studies considering multipollutant nature of air pollution and additional outcomes are needed to demonstrate the underlying mechanism.
Introduction
Exposure to ambient air pollution (AP) increases morbidity and mortality and contributes substantially to the global burden of disease [1]. AP increases the risk of respiratory and cardiovascular diseases, strokes, allergic diseases, diabetes, and autoimmune diseases in adults [2-5]. AP is generated mainly from fossil fuel combustion, industrial processes, construction work, cigarette smoking, and consumer products and is naturally produced by wildfires, volcanoes, and thunderstorms [6]. The components of AP are complex and mixed with natural or artificial substances and contain large volumes of gases, liquid droplets, or solid particles. Gaseous components of AP include ozone (O3), nitrogen dioxide (NO2), sulfur dioxide (SO2), carbon monoxide, and carbon dioxide. Particulate matter (PM) includes dust, soil, organic acids, and metals, and some of these compounds have similar effects to endocrine-disrupting chemicals (EDCs) [6]. PM is categorized based on particle size –PM10 (smaller than 10 μm), PMcoarse (ranging from 2.5 μm to 10 μm), PM2.5 (smaller than 2.5 μm), and ultrafine PM (smaller than 0.1 μm). Traffic-related AP contributes significantly to outdoor AP, especially in urban settings, and is comprised of nitrogen oxides (NOx) of nitric oxide (NO) and NO2 and PM. In children, there is growing evidence that AP can affect the endocrine system. In this review, we discussed the effects of ambient APs on childhood endocrinologic disorders and possible associated mechanisms.
Association between AP and childhood endocrinologic disorders in human studies
1. Type 1 diabetes mellitus
Type 1 diabetes mellitus (T1DM) is an autoimmune disease characterized by destruction of insulin-producing pancreatic islet beta cells. The exact etiology is understood incompletely, although disease development is influenced by both genetic and nongenetic factors, including infections, early infant diet, gut microbiome, and vitamin D deficiency [7]. It recently has been suggested that environmental chemicals and AP are associated with development of T1DM [8]. Previous case-control studies of children with T1DM showed a relationship between development of T1DM and concentrations of O3 [9] and PM10, especially in children younger than 5 years [10]. Research has found that households with diabetes were more likely to be exposed to secondhand smoke than nondiabetic households [9].
Human studies of the relationship between ambient AP and T1DM in pediatric patients have investigated age at onset, incidence, or disease exacerbation of T1DM (Table 1). Three studies that evaluated the effect of prenatal ambient AP exposure showed inconsistent results [11-13]. A Swedish observational study found that NOx exposure during the third trimester of pregnancy was associated with development of T1DM in children [11]. However, in recent studies, exposure to NO or NO2 had no significant effect on the incidence of T1DM [12,13]. Results of the effects of maternal O3 exposure consistently showed that O3 exposure was associated with increased incidence of T1DM in children [11-13]. However, the associations between PM10 [13] and PM2.5 exposure [12,13] during gestation and incidence of T1DM in children were not significant.
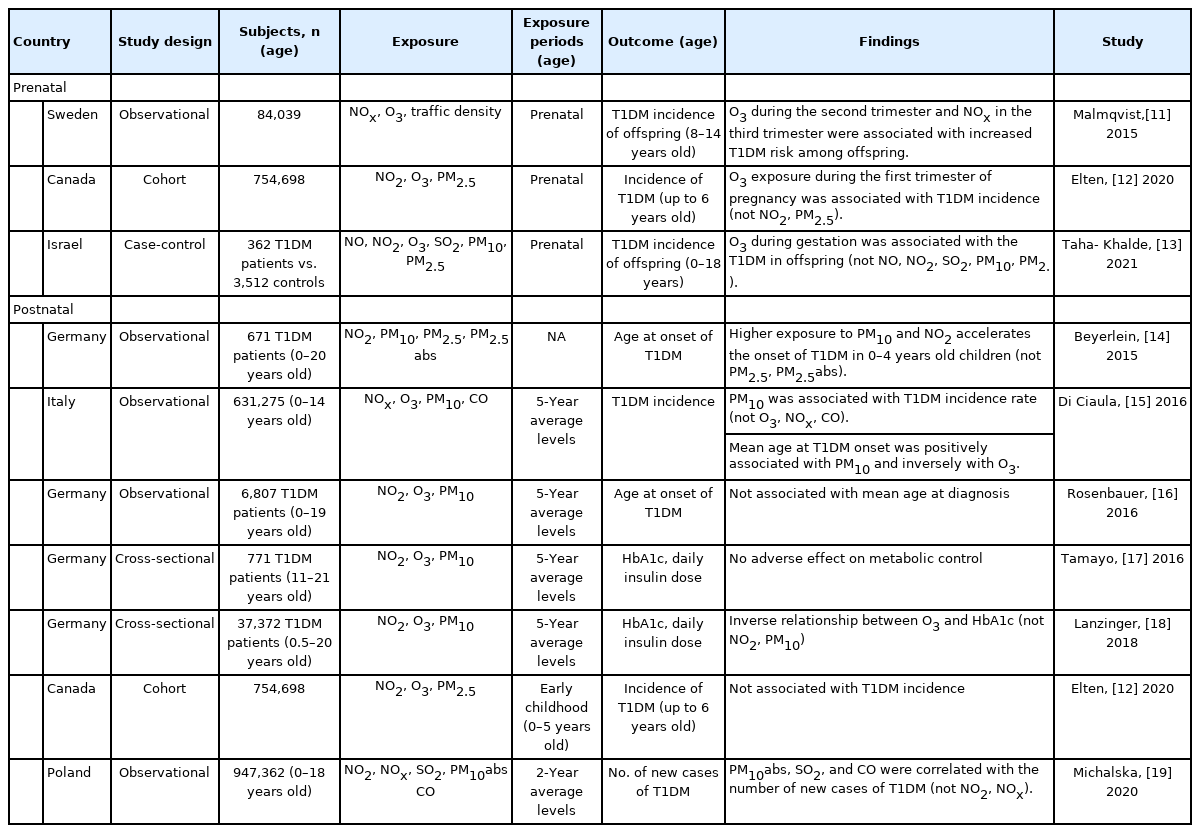
Human studies investigating the relationship of ambient air pollution and type 1 diabetes in children
Results from postnatal exposure studies that assessed air pollutants were inconsistent [14-19]. Most previous studies showed no relationship between exposure to NOx or NO2 during the postnatal period and incidence [12,15,19], or age at onset [16] of T1DM or serum hemoglobin A1c (HbA1c) level among patients with T1DM [17,18], with the exception of one study reporting that greater exposure to NO2 accelerated onset of T1DM in early childhood (0–4 years of age) [14]. The increased annual mean concentration of O3 during childhood or adolescence accelerated the mean age at onset of T1DM [15], although no direct relationship was observed [16]. Other studies reported no association [17] or an inverse association [18] with serum HbA1c level among patients with T1DM, suggesting a therapeutic effect of O3 through blood glucose reduction, because moderate oxidative stress induced by O3 activated both free antioxidants and antioxidative enzymes [20]. Postnatal PM10 exposure was associated with the increased incidence of T1DM in children [15,19]. The effect on mean age at onset of T1DM of PM10 exposure varied from no association [16] to younger [14] or older [15]. The annual mean concentration of PM10 was not related to serum HbA1c level in patients with T1DM [17,18]. Further, PM2.5 exposure was not related to the incidence of T1DM [12] or the age of onset in patients with T1DM [14].
2. Childhood obesity
Childhood obesity can be promoted by multiple factors, primarily due to an imbalance between energy intake and consumption. There is growing evidence that environmental chemical exposure can act as an "obesogen" and contribute to excessive weight gain [21]. Previous studies revealed that maternal exposure to combustion-derived polycyclic aromatic hydrocarbons [22] and cigarette smoke during pregnancy [23] was associated with an increased risk of childhood obesity.
To date, results from human epidemiological studies on the relationship between ambient AP and childhood obesity have been mixed and inconclusive (Table 2). Further, the results of research on the effects of prenatal exposure to ambient AP on infant or child weight gain were inconsistent [24-30]. PM2.5 exposure during gestation was not associated with weight gain in infancy [24] or adiposity [25,30] or body mass index (BMI) trajectory [28,29] during early- or midchildhood; however, a positive association between prenatal PM2.5 exposure and childhood overweight or obesity [26] or adiposity [27] was reported. While most previous studies have set the specific exposure time and investigated the impact of the average concentration during that period, one cohort study used Bayesian distributed lag interaction models to identify prenatal periods that could be sensitive windows influencing childhood obesity by sex [27]. This research suggested that increased exposure to PM2.5 in midpregnancy was associated with increased fat mass and higher BMI z-score (body size) among boys, and higher exposure to PM2.5 from early-to-mid pregnancy was associated with increased waist-to-hip ratio (body shape). Moreover, another cohort study simultaneously assessed the impact of 4 air pollutants (PM10, SO2, NO, and NO2) during prenatal and postnatal periods using a multipollutant model to account for collinearity between pollutants and exposure periods and showed that higher SO2 in utero and in childhood was associated with lower BMI, while higher NO2 in childhood was associated with higher BMI among boys [30].
Different studies have shown a significant association between postnatal exposure to ambient AP and BMI [25,28,30-34] and the risk for becoming overweight and obese [26,35-41], while no relationship with BMI [42] or a negative association with BMI or obesity-related parameters [25,30] has been reported. Longitudinal U.S. cohort studies have shown a positive association between higher traffic density within 150 m around a residence and BMI at 18 years of age [31], although the perimeter was not associated with early- and mildchildhood obesity-related parameters [25,42]. Most studies have shown a positive relationship between postnatal NOx or NO2 exposure and obesity-related parameters and reported a greater increase in BMI [28,30,32-34] and attained BMI at 10 years [28,32] and 18 years [33] of age and a higher risk of being overweight or obese [35-37,39-41], although an Italian cohort study did not report any significant results [42].
In regard to O3 exposure, a positive association with overweight or obesity was reported in 2 Chinese cross-sectional studies [35,39]. PM10 exposure was positively associated with risk of being overweight or obese in childhood in some studies [35,39,41], but no relationship was found between PM10 exposure and obesity-related parameters or the risk of being overweight or obese during childhood in other studies [30,36,39,42]. Effects of postnatal PM2.5 exposure were associated with a higher BMI [34,40] or risk of being overweight or obese [37,38,41]; however a negative [25] or no association [36,42] with obesity-related parameters also was observed. Several studies have shown a sex difference [27] or a strong association between ambient AP and obesity in boys compared with girls [30,39,40], which might be linked to sex differences in biological responses to environmental chemicals and social and behavioral factors. Recent cross-sectional studies showed a significant association of increased risk of obesity in school-aged children by measuring exposure to ambient AP based on the nearest air monitoring station at school instead of home, where children spend most of their time [37-40].
3. Insulin resistance
An increase in the prevalence of type 2 diabetes mellitus (T2DM) is a global concern for mortality and disability in adults [43]. In addition to traditional risk factors such as poor diet, low physical activity, and socioeconomic status, recent studies have suggested that ambient AP exposure can contribute to T2DM development. Although several systematic reviews and meta-analyses have revealed a relationship between ambient AP exposure and T2DM risk in adults [44-47], no reports have assessed the risk of T2DM due to ambient AP exposure in children. Several studies have evaluated the effects of AP and the association with diabetes development in children and insulin resistance.
Three reports investigating the effect of prenatal exposure to ambient AP on insulin resistance showed inconsistent results (Table 3) [48-50]. Prenatal exposure to NO2 was not associated with cord plasma insulin level in infants [48], which might be a risk factor of metabolic disease later in life. However, the exposure paradoxically was associated with fasting glucose, insulin, and homeostatic model assessment for insulin resistance (HOMA-IR) in adolescents between 10–15 years of age [50]. Higher prenatal PM2.5 and PM10 exposures were associated with increased cord plasma insulin level [48], and prenatal and perinatal PM2.5 exposure was associated with an annual increase in serum HbA1c level in girls from 4–5 years to 6–7 years of age [49]. These 2 studies commonly reported that the second trimester of pregnancy was an exposure window associated with increased serum HbA1c level later in childhood.
Most previous studies have reported a positive association of higher rates of exposure to NO2 during childhood and adolescence with increased HOMA-IR [51,52] or insulin resistance [34,53] and increased fasting glucose level [53,54], with the exception of one report that showed a negative association [50]. Exposure to PM10 was associated with increased HOMA-IR in 2 German studies [51,52], although exposure was not associated with fasting glucose level in a Chinese study [54]. Results from studies on exposure to PM2.5 have ranged from negative [25] or no relationship with HOMA-IR [51,52], to a positive association with lower insulin sensitivity [53]. Two studies in the United States that included overweight or obese children used the frequently sampled intravenous glucose tolerance test and revealed that higher NO2 and PM2.5 were associated with higher insulin resistance and secretion, as measured by higher glycemic values [34,53], and a faster decline in insulin sensitivity during follow-up, independent of adiposity [34]. These findings suggest that increased AP exposure is an independent risk factor for β-cell exhaustion. Only one intervention and prospective study was conducted in adolescents who underwent laparoscopic adjustable gastric banding due to severe obesity. That study found that increased exposure to NO2 attenuated the magnitude of HbA1c reduction, a known metabolic benefit of gastric banding [55].
4. Thyroid dysfunction
Several environmental chemicals have structures similar to those of thyroid hormones. These chemicals include polychlorinated biphenyls, triclosan, polybrominated diphenyl ethers, and bisphenol A and can reduce circulating levels of thyroid hormone by interfering with thyroid hormone metabolism, transport, and clearance [56]. Findings from previous studies suggest that airborne persistent organic pollutants [57], cadmium [58], and exposure to active and passive cigarette smoke [59] can affect thyroid hormone regulation and function in neonates and adults.
A few human studies on the impact of PM pollution exposure on thyroid function have been conducted, although these studies have focused on the relationship between maternal exposure and neonatal thyroid function (Table 4) [60-63]. Maternal exposure to PM2.5 in the third trimester was inversely associated with cord blood thyroid-stimulating hormone (TSH) level and the free thyroxine (T4)/free triiodothyronine (T3) ratio and was positively associated with cord blood free T3 [60], but no association between maternal PM2.5 exposure in the first trimester and neonatal TSH level was found [62]. These 2 studies identified cord blood free T4 [60] and maternal free T4 in the second trimester [62] as a partial mediator that linked prenatal PM2.5 exposure and birth weight of newborns. Another study evaluated the susceptible prenatal window period in which PM2.5 exposure at the end of the first trimester and PM10 exposure throughout most of the pregnancy were associated with higher total newborn T4 concentration in heel-prick blood spot test [61]. A cross-sectional study in China showed that high PM2.5 exposure during pregnancy was associated with increased incidence of congenital hypothyroidism in offspring [63]. However, no studies have investigated the association between postnatal PM exposure and thyroid function in childhood or adolescence.
5. Pubertal development
Adiposity and exposure to EDCs have been suggested as important factors in the association between environmental factors and pubertal onset, particularly with respect to the current decline in the average age at onset of puberty in girls [64]. For example, early life tobacco exposure [65] or secondhand and prenatal smoke exposure [66] is associated with earlier pubertal maturation.
A few epidemiological studies investigating AP and pubertal development in children have shown inconsistent results (Table 5) [67-70]. An epidemiological study in Hong Kong showed differences between boys and girls concerning the type of AP and window time, which was related to later pubertal development, and the results were based on multipollutant analysis [67]. Higher PM10 exposure in utero and in infancy lowered the pubertal stage among girls, whereas higher SO2 and NO2 exposure in utero and during childhood lowered the pubertal stage among boys. In contrast, girls that lived within 150 m from major roads or highways developed pubic hair several months earlier than those that lived further away [68]. Moreover, exposure to a higher concentration of PM10 in the pre-menarche period was associated with lower menarche age in Korean adolescents, and the risk of early menarche was higher when the exposure period was shorter, indicating that the neuroendocrine system becomes susceptible to PM10 exposure at the time of menarche [69]. However, no relationship between air pollutants (NO2, O3, PM10, and PM2.5) and serum sex hormone levels in 10-year-old children was reported [70].
Possible mechanisms
1. The immune system and inflammatory responses
The mechanism by which air pollutants contribute to endocrinologic disorders is not known, although altered immune responses and inflammatory reactions have been suggested as possibilities [71]. Inhaled AP comes into contact with alveolar macrophages and induces proinflammatory cytokine production as well as oxidative stress. These cytokines can spill over into systemic circulation and affect distant tissues, promoting autoimmune responses and metabolic dysfunction [71]. PM components such as transition metals, lipopolysaccharides, and O3 can infiltrate into the systemic vasculature and activate toll-like receptors [72]. Signal transduction, including transcription factor nuclear factor kappa B, is activated and promotes the production of proinflammatory cytokines (interleukin [IL]-4, IL-6, IL-8, and tumor necrosis factor [TNF]-α) [73,74], which leads to chronic inflammation and low-grade oxidative stress in the body. Particularly, PM2.5 modulates cytokine production and changes the balance between TNF-α and the production of anti-inflammatory IL-10 molecules in adolescents [75]. Increased IL-10 and reduced TNF-α levels serve as a biomarker for T helper 1 cell-mediated immune suppression and exacerbation of T helper 2-mediated humoral immune responses, contributing to the development of autoimmune diseases such as T1DM.
2. The neuroendocrine system
The neuroendocrine system can be important in AP-induced endocrine dysfunction. An experimental study revealed that exposure to prenatal diesel exhaust induced direct neuroinflammation and neuronal structural changes in the feeding centers of the hypothalamus and increased vulnerability to a high-fat diet and weight gain later in life [76], suggesting direct alteration of the central nervous system. AP also can activate stress-responsive regions in the brain through the sympathetic efferent and the hypothalamus-pituitary-adrenal axes [77]. For example, O3 inhalation evokes lung inflammation that induces the activation of nucleus tractus solitarius neurons through the vagus nerves and promotes neuronal activation in stress-responsive regions of the central nervous system in mice.78) O3 exposure also increased circulating corticosterone and cortisol levels in humans [79]. These results show that increased neuronal stress response can affect metabolic regulation.
3. Placental development and epigenetic modulation
Environmental stimuli or challenges during critical periods can alter placental development to the extent that the placenta could adapt by alternating transporter expression and activity to maintain fetal growth or by epigenetic regulation of placental gene expression, resulting in detrimental consequences later in life [80]. Inadequate placental perfusion affects fetal growth of the endocrine system in utero. AP exposure during pregnancy contributes to an anti-angiogenic profile, which could decrease placental weight [81] and is associated with increased inflammatory markers [82,83].
The epigenetic repression and activation of gene transcription are affected by environmental stimuli, such as nutrition, light, and endocrine disruptors [84]. There is increasing evidence that epigenetic mechanisms play an important role in the development of T1DM [85,86] and also in neuroendocrine system regulation, which can impact the timing of puberty [84,87]. In addition, a recent study showed that in utero exposure to polycyclic aromatic hydrocarbons induces offspring obesity by hypomethylation of peroxisome proliferator-activated receptorgamma and by subsequent activation of various genes associated with adipogenesis in adipose tissue of the offspring. Exposure to PM also induces deoxyribonucleic acid methylation [88], which could be a partial mediator between PM and adverse health outcomes.
4. Obesity and insulin resistance
The effects of increased AP exposure on the development of obesity and insulin resistance are complex and multifactorial. Excess AP is associated with decreased outdoor activities and reduced energy expenditure, which increases the likelihood of obesity. Alternations in mitochondrial number and size, downregulated brown adipocyte-specific genes in thermogenesis, and energy expenditure also are induced by PM exposure [89]. In addition, exposure to AP can alter the basal metabolism, including white adipose tissue inflammation, inhibition of lipolysis, and redistribution of adipose tissue in the viscera [89-92], playing a key role in the development of insulin resistance, diabetes, and systemic inflammatory effects. Endothelial dysfunction after AP exposure [93,94] is implicated in reduced peripheral glucose uptake. O3 also creates free oxygen radicals that directly contribute to beta-cell damage [95].
5. Thyroid system
Research has indicated that oxidative stress, inflammatory status, alterations to the neuroendocrine system, and inadequate placental adaptation can affect the thyroid. For example, increased glucocorticoid activity markers inhibit TSH release. Increased thyroid-binding globulin was observed after exposure to cigarette smoke, which could be transferred via the placenta, and can lead to higher total T4 level and lower free T4 level [96]. Cigarette smoke was associated with stimulated conversion of T4 to T3 by activities that promoted type 2 deiodinase in tissues, leading to decreased free T4 level and increased free T3 level [97]. A recent experimental study in female rats found that PM2.5 exposure could reduce circulating thyroid hormone levels by interrupting thyroid hormone biosynthesis, biotransformation, and transport; inducing oxidative stress and inflammatory responses; and ultimately activating the hypothalamic-pituitary-thyroid axis and inducing the production of hepatic transthyretin [98].
6. Pubertal development
The mechanisms that link AP and pubertal development or sex hormones have not been investigated, although they are expected to mimic the effects of EDCs. These effects could be caused by several mechanisms that impact puberty either peripherally or centrally; the agents could act as agonists of estrogen receptors or antagonists of androgen receptors and as obesogens, which alter the metabolic and peripheral hormones and can affect genes or the hypothalamic-pituitary-gonad axis [99].
Conclusion
Human studies provide considerable evidence of short-and long-term exposures to ambient APs, such as PM, NO2, and NOx, which affect the endocrine system and contribute to the development of childhood T1DM, obesity, and insulin resistance, although conflicting results have been reported. However, there is little evidence on the effect on thyroid function on onset of puberty. Altered immune response, oxidative stress, neuroinflammation, inadequate placental development, and epigenetic modulation are some of the underlying factors that have been identified and investigated. However, it is difficult to demonstrate causality because results from human studies are heterogeneous due to different study designs, timing and degree of exposure, methodology of exposure, and outcome measurements. Additionally, ambient APs are composed of various microscopic solids or liquid droplets and EDCs, and the extent to which airborne EDCs contribute to the overall burden on the human body is unknown. To further understand the mechanisms that link AP and the risk of endocrine disorders in children, future studies should consider the multipollutant nature of the mixture and the varying chemical composition, which could lead to different toxicities according to sex or susceptible window. Studies on additional outcomes such as changes in metabolomics and the microbiome in the intestine and central nervous system are needed to evaluate the biological pathway. Future research can help prevent environmental toxicity and improve treatment approaches for endocrine disorders.
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.




