Effects of gonadotropin-releasing hormone agonist treatment on final adult height in boys with idiopathic central precocious puberty
Article information
Abstract
Purpose
There are few reports on the therapeutic effects of gonadotropin-releasing hormone agonists in boys with central precocious puberty, and studies reported in Korea are very rare. We aimed to assess the significance of clinical factors and the effects of gonadotropin-releasing hormone agonist treatment on final adult height in boys diagnosed with central precocious puberty.
Methods
We retrospectively evaluated the medical records of 18 boys treated for idiopathic central precocious puberty between 2007 and 2018 at Chosun University Hospital. Gestational age, birth weight, and parental height were assessed at the initial visit. Chronological age, bone age, bone age/chronological age ratio, height and height standard deviation scores, predicted adult height, body mass index, and hormone levels were assessed during the treatment period.
Results
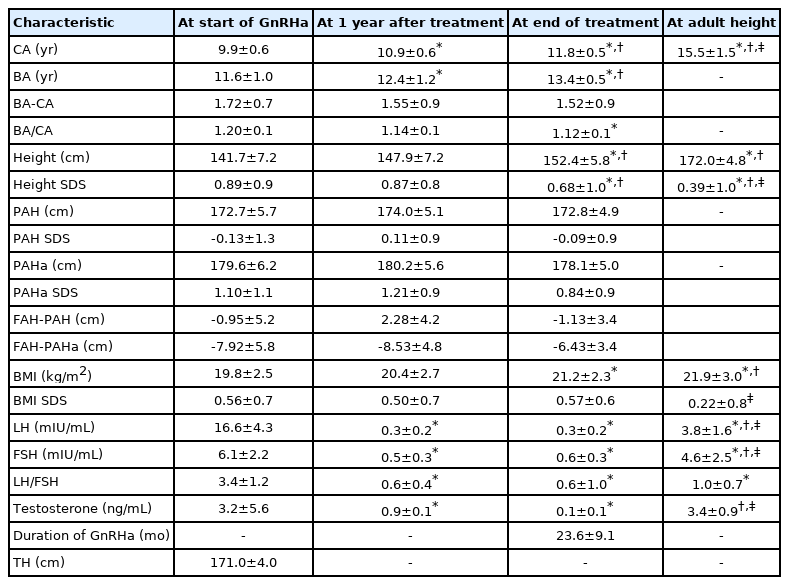
At the time of diagnosis, the chronological age was 9.9±0.6 years, the bone age was 11.6±1.0 years, and the bone age/chronological age ratio was 1.20±0.1. The bone age/chronological age ratio decreased significantly to 1.12±0.1 at the end of treatment (P<0.05). The luteinizing hormone/follicular stimulating hormone ratios were 3.4±1.2, 0.6±0.4, and 0.6±1.0 at the start of treatment, after 1 year of treatment, and at the end of treatment, respectively. After gonadotropin-releasing hormone agonist treatment, the final adult height reached 172.0±4.8 cm compared to the target height range of 171.0±4.0 cm.
Conclusions
In boys with central precocious puberty, gonadotropin-releasing hormone agonist treatment improved growth potential.
Highlights
GnRHa is a standard treatment for central precocious puberty in both boys and girls but, there are few reports on the effects of GnRHa in boys with central precocious puberty. So, this study aimed to analyze the significance of clinical factors and their effects on final adult height following GnRHa treatment diagnosed with central precocious puberty.
Introduction
The incidence of precocious puberty has recently increased along with the accelerated onset of puberty [1]. Central precocious puberty refers to conditions where activation of the hypothalamic-pituitary-gonadal axis leads to breast and testicular development before age 8 years in girls and 9 years in boys [2]. Although the prevalence of central precocious puberty in boys is approximately 10 to 15 times lower than that in girls [3,4], the incidence of central precocious puberty caused by organic conditions of the central nervous system is much higher in boys (40% to 90%) than in girls (only 8% to 33%). Therefore, brain magnetic resonance imaging (MRI) must be performed in boys with central precocious puberty to exclude organic causes [5,6].
Early puberty accelerates growth and promotes bone maturation, resulting in early fusion that causes a decrease in final adult height (FAH) [7-9]. Therefore, gonadotropin-releasing hormone agonists (GnRHa) are prescribed for children diagnosed with central precocious puberty to suppress the secretion of sex hormones, inhibit bone maturation, and improve FAH [7,8].
While there have been several studies on the effects of GnRHa treatment on clinical factors and FAH in girls with central precocious puberty, studies on the effects of long-term GnRHa treatment and FAH in boys with central precocious puberty are scarce, and studies reported in Korea are very rare. Therefore, the purpose of this study was to analyze the significance of clinical factors and their effects on FAH following GnRHa treatment in boys diagnosed with central precocious puberty.
Materials and methods
1. Study design and patients
This was a retrospective study of 18 boys diagnosed with and treated for central precocious puberty at the Department of Pediatrics of Chosun University Hospital between January 2007 and December 2018 and whose FAH could be assessed. Gestational age, birth weight, and parental height were assessed at the initial visit. Chronological age (CA), bone age (BA), BA/CA ratio, height, body mass index (BMI), height standard deviation score (SDS), BMI SDS, predicted adult height (PAH), PAH SDS, luteinizing hormone (LH) level, follicle-stimulating hormone (FSH) level, LH/FSH ratio, and testosterone level were measured at three time points: the start of GnRHa treatment, after 1 year of treatment, and at the end of treatment.
The diagnostic criteria of central precocious puberty were (1) testicular volume of 4 mL or more before age 9 years and (2) peak LH level of 5 mIU/mL or more in the GnRH stimulation test. Patients with congenital adrenal hyperplasia and gonadal tumors were excluded from this study as these conditions can cause hypersecretion of male hormones. Brain MRI was performed to rule out central nervous system tumors. We excluded 6 children in whom an underlying organic brain lesion was found in brain MRI (microadenoma n=4. Rathke’s cyst n=1, harmatoma n=1).
2. Measurements
BA was measured using the Greulich-Pyle method by taking simple radiographs of the left hand and wrist [10]. Testicular volume was measured using a Prader orchidometer. Target height (TH) was defined as the midparental height, which was calculated by adding 6.5 cm to the average parental height. Bayley-Pinneau (BP) average tables and BP-advanced tables were used to measure PAH and PAHa, respectively [11]. FAH was defined as the height measured when BA ≥16 years and growth rate<1 cm/yr. The standard growth chart for children and adolescents published by the Korean Pediatrics Association in 2017 was used [12].
3. Statistical analysis
The results were expressed as the mean value±standard deviation, and a Friedman test was performed to evaluate the significance of the difference in the mean value of the clinical factors according to the treatment period of all 18 children. Spearman correlations analysis was performed to assess the relationship between the FAH and PAH assessed using the average and advanced tables of the BP method during the treatment period. For factors that can affect FAH, simple linear regression and multiple linear regression with stepwise variable selection were used. All statistical analyses were performed using IBM SPSS Statistics ver. 24.0 (IBM Co., Armonk, NY, USA).
Results
1. Patient characteristics
Clinical factors according to the period of GnRHa treatment in a total of 18 boys are shown in Table 1. The CA and BA at the start of GnRHa treatment were 9.9±0.6 years and 11.6±1.0 years, respectively. After 1 year of treatment, the CA was 10.9+0.6 years, and the BA was 12.4±1.2 years. At the end of treatment, the CA and BA were 11.8±0.5 years and 13.4±0.5 years, respectively. The BA/CA ratios were 1.20±0.1, 1.14±0.1, and 1.12±0.1 at the start of treatment, after 1 year of treatment, and at the end of treatment, respectively. BA/CA ratio reduction was statistically significant at diagnosis and the end of treatment (P<0.05), suggesting that bone maturation was inhibited by GnRHa treatment. The BA–CA difference decreased during the treatment period, but there was no statistical significance. The mean duration of GnRHa treatment was 23.6±9.1 months.
The TH of all 18 children was 171.0±4.0 cm, and the FAH after GnRHa treatment was172.0±4.8 cm. The LH/FSH ratio at the time of diagnosis was 3.4±1.2, and it was significantly decreased to 0.6±0.4 and 0.6±1.0 after 1 year of treatment and at the end of treatment, respectively (P<0.05). The testosterone level at the time of diagnosis was 3.2±5.6 ng/mL, and it significantly decreased to 0.9±0.1 ng/mL after 1 year of treatment (P<0.05).
2. Relationship of PAH, PAHa, and FH according to treatment period
The correlation between FAH and height, PAH, PAHa according to treatment period is shown in Fig. 1. At the start of GnRHa treatment (Fig. 1A), the FAH correlated with height (R2=0.2596, P<0.05), PAH (R2=0.2697, P<0.05), PAHa (R2=0.2204, P<0.05). Similarly, at 1 year after treatment (Fig. 1B) and at end of treatment (Fig. 1C), the FAH correlated with height (R2=0.2404, P<0.05), PAH (R2=0.4060, P<0.05), PAHa (R2=0.3373, P<0.05) and (R2=0.4900, P<0.05), PAH (R2=0.5708, P<0.05), PAHa (R2=0.5714, P<0.05), respectively. During the treatment period, a significant correlation was found between FAH and height, PAH, and PAHa. At the end of treatment, PAH and PAHa showed a high correlation with FAH.
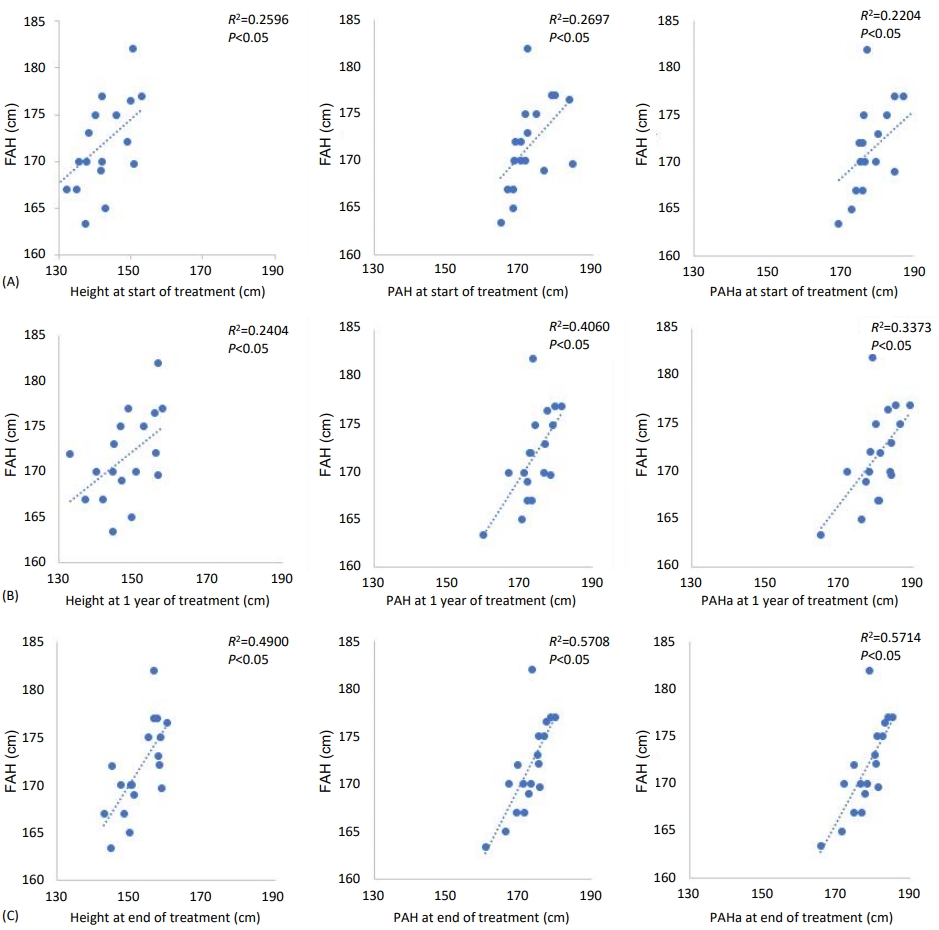
Correlation between FAH and height, PAH, PAHa during treatment period. At start of treatment (A), 1 year after treatment (B), and end of treatment (C). FAH, final adult height; PAH, predicted adult height-calculated with average table of Baley-Pinneau (BP) method; PAHa, predicted adult height-calculated with advanced table of BP method.
At the start of GnRHa treatment, the PAH and PAHa were 172.7±5.7 cm and 179.6±6.2 cm, respectively, which were higher than the mean TH of 171.0±4.0cm. PAH and PAHa after 1 year of treatment were increased relative to that at the start of treatment to 174.0±5.1 cm and 180.2±5.6 cm, respectively. At the end of treatment, PAH was 172.8±4.9 cm and PAHa was 178.1±5.0 cm. The FAH was 172.0±4.8 cm, which was within the TH of 171.0±4.0 cm.
3. Clinical factors influencing FAH
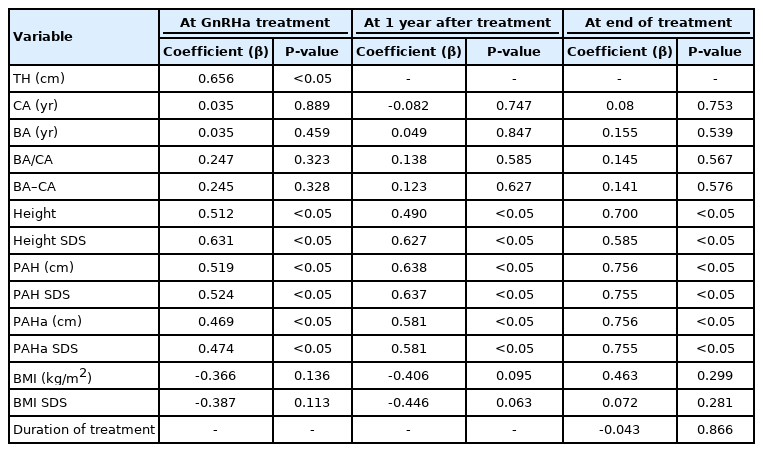
We performed a simple linear regression analysis on the factors that influence the FAH (Table 2). At the start of GnRHa treatment, TH, height, height SDS, PAH, and PAHa were positively correlated with FAH (β=0.656, 0.512, 0.631, 0.519, 0.524, 0.469, and 0.474, respectively; all P<0.05). After 1 year of treatment, height, height SDS, PAH, and PAHa remained significant influencing factors (β=0.490, 0.627, 0.638, 0.637, 0.581, 0.581, respectively; all P<0.05). At the end of treatment, height, height SDS, PAH, SDS of PAH, PAHa, and PAHa SDS had significant positive correlations with FAH (β=0.700, 0.585, 0.756, 0.755, 0.756, and 0.755, respectively; all P<0.05). In multiple linear regression analysis using stepwise variable selection based on factors that were significantly correlated with the FAH during the treatment period, only PAHa at the end of treatment was found to be significantly correlated with the FAH (R2=0.571, P<0.05) (Table 3).
Discussion
In this study, we analyzed the effect of GnRHa treatment on FAH in boys diagnosed with central precocious puberty. Precocious puberty caused by organic conditions is more frequent in boys than in girls, and the cause of central precocious puberty in boys must be identified [13]. The incidence of central precocious puberty in boys has increased over the past 10 years and has been reported to be associated with an increase in idiopathic precocious puberty. In a study of 100 boys with central precocious puberty from 2003 to 2014, 74% had idiopathic disease, and the remaining 26% of cases were caused by organic conditions. Moreover, when the authors divided the cases into 2-year unit (i.e., 2003–2005, 2006–2008, 2009–2011, and 2012–2014), the proportion of children with idiopathic central precocious puberty was shown to be gradually increasing over time (33.3%, 64.7%, 80.6%, and 81.6%, respectively) [14].
GnRHa is a standard treatment for central precocious puberty in both boys and girls. It is known to suppress the secretion of sex hormones to slow puberty and suppress rapid bone fusion to achieve FAH within the TH range [15,16]. However, there have been fewer studies on the effects of long-term GnRHa treatment in boys with central precocious puberty than in girls. Existing studies published abroad also had a limited sample size. Moreover, there are very rare studies in Korea on the effects of GnRH treatment on clinical factors and FAH in boys with central precocious puberty. In a multicenter study conducted in Italy involving 45 boys with precocious puberty, 27 (60%) had idiopathic disease and 18 children (40%) had central nervous system lesions. Hamartoma was the most common central nervous system lesion, followed by neurofibromatosis (5 children), central nervous system malformation (5 children), and ependymoma (2 children). The basal LH level and the peak LH/FSH ratio were higher in children with organic lesions, but the difference was not significant [17]. In a study of 22 girls and 11 boys with idiopathic central precocious puberty, both boys and girls showed a significant decrease in the BA/CA ratio during treatment, and PAH was increased. In girls, the PAH at the start of treatment was 155.2±4.7 cm, and this significantly increased to 160.5±6.1 cm at the end of treatment. Similarly, the PAH was 168.3±2.12 cm in boys at the start of treatment and was significantly increased to 179.0±5.0 cm at the end of treatment. The FAH was 158.5±5.3 cm and 179.0±5.0 cm for girls and boys, respectively, which was significantly higher than the pretreatment PAH in both groups [18].
In a study of 12 boys with central precocious puberty published in Italy, the CA and BA at the time of diagnosis were 7.6±0.9 years and 10.9±1.7 years, respectively. The TH was 174.2±2.9 cm, and the PAH at the time of diagnosis was 169.9±4.2 cm. During treatment, the PAH significantly increased to 180.7± 6.0 cm. Furthermore, the FAH was 176.1±6.1 cm, which was increased as compared with TH and PAH at the time of diagnosis. Height SDS at the end of treatment was identified to be an influencing factor of FAH [19]. In a study of 98 children with precocious puberty, the FAH of 18 boys was 171.1±8.7 cm, which was significantly increased from the PAH of 156.1±14.2 cm at the time of diagnosis [20]. In the current study, the FAH was 172.0±4.8 cm, which was higher than the TH of 171.0±4.0 cm. However, during the treatment period, PAH and PAHa were increased compared to the TH of 171.0±4.0 cm. Considering that in boys, bone maturity appears in the latter half of sexual maturity compared with girls, there is a time difference before the increased sex hormone concentration at the time of diagnosis of precocious puberty is reflected in BA. Thus, it is thought that BA is less evolved at the time of diagnosis, which leads to exaggerated PAH and PAHa as assessed using the BP method [21.22]. Compared to previous studies showing that untreated patients might lose their growth potential up to 10 cm [18], our study in boys with GnRHa treatment recovered growth potential beyond the range of TH. Previous studies showed that the influencing factors of FAH after GnRHa treatment in girls with central precocious puberty included young CA at the time of diagnosis, height SDS at the start and end of treatment, and duration of treatment [23-25]. In our previous study of 69 girls diagnosed with and treated for central precocious puberty, the significant factors related to FAH were TH, height at the start of GnRHa treatment, height SDS, PAH, and height SDS at the end of treatment [26]. The few studies on the influencing factors of FAH following GnRHa treatment in boys identified TH and height at the end of GnRHa as important predictive factors [19]. In the current study, PAHa at the end of treatment was positively associated with FAH.
Interestingly, in our study, the level of FAH was similar to the PAH based on the BP method average table. It is well known in the literature that when predicting adult heights via the BP method, the prediction may overestimate FAH in pediatric patients with normal or advanced BA and PAH, which does not greatly differ regardless of treatment, is more appropriate in predicting FAH in pediatric patients with CPP. Additionally, caution should be taken in predicting FAH in practice because the prediction using the BP method during GnRHa treatment or end of treatment overestimates FAH [27.28]. That is, in comparison to normal puberty, gonadotropin and sex hormone levels elevate more rapidly after treatment is complete, arriving at the maximum level sooner, and the children reach their FAH earlier. Hence, the FAH during treatment and afterwards may be smaller [29]. Likewise, in our study, the genetic potential to reach TH was recovered in FAH, but the height was found to be smaller than PAH and PAHa at the time of treatment completion and rather closer to the PAH based on the average table. However, in this study we identified PAHa at the end of treatment as a factor influencing FAH. Rather than interpreting the finding as showing a value close to actual FAH, we believe it more appropriate to interpret the factor as influencing growth to FAH by recovering growth potential after treatment to help increase FAH in the children.
As previously mentioned, there are few papers dealing with the treatment effect of GnRHa on clinical factors and FAH in Korean male CPP patients. In this respect, our study will be helpful in future clinical applications. However, this study was also conducted on a small number of subjects with a relatively short follow-up period. Therefore, long-term studies with a larger sample size are needed to validate our findings.
In conclusion, GnRHa treatment can help boys with precocious puberty reach FAH near the TH range.
Notes
Ethical statement
This study was approved by the Institutional Review Board (IRB) of Chosun University Hospital (IRB No. 2020-04-006). This study was retrospective and was, therefore, exempted from need for informed consent.
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Funding
This study was supported by a research fund received from Chosun University Hospital 2019.