Risk of cancer in patients treated with recombinant human growth hormone in childhood
Article information
Abstract
Recombinant human growth hormone (GH) has been in use for over 30 years, and its indications have gradually expanded from the classical replacement therapy in GH deficiency (GHD) to pharmacological therapy in patients with normal GH secretion. The insulin-like growth factor-I (IGF-I ) is closely GH dependent and is the effector of GH biological actions in peripheral tissues. Since IGF-I has potent mitogenic and antiapoptotic effects, the use of GH, especially outside GHD, has raised safety concern regarding cancer risk. The results of experimental, epidemiological and observational studies are not univocal and a number of biases and confounders affect the interpretation of data. The aim of this review is to critically review the data linking GH therapy during childhood with cancer risk, highlighting strengths and weaknesses of the available evidence.
Introduction
Recombinant human growth hormone (r-hGH) was introduced in the market in 1985 for the treatment of children with growth hormone deficiency (GHD). Thereafter, due to the virtually unlimited supply of r-hGH, the indications for its use have progressively expanded worldwide to include patients with normal growth hormone (GH) secretion (Table 1). Therefore, r-hGH therapy has changed over the years from a classical replacement therapy to a pharmacological treatment. The use of r-hGH in patients with normal GH secretion has raised concerns about the safety of such therapy. The final mediator of the growth promoting action of GH is insulin-like growth factor-I (IGF-I), which exerts potent antiapoptotic and mitogenic activity in all cells of the organism and is expressed and secreted from many different types of cancer cells. However, while there is strong evidence for a role of the GH-IGF axis in the development, maintenance, and spread of tumors based on experimental data obtained in cellular and animal models, such evidence is weak in humans [1].
The aim of this review is to provide an up-to-date critical review of data linking GH therapy during childhood with cancer risk, highlighting strengths and weaknesses of the available evidence.
The GH – IGF axis
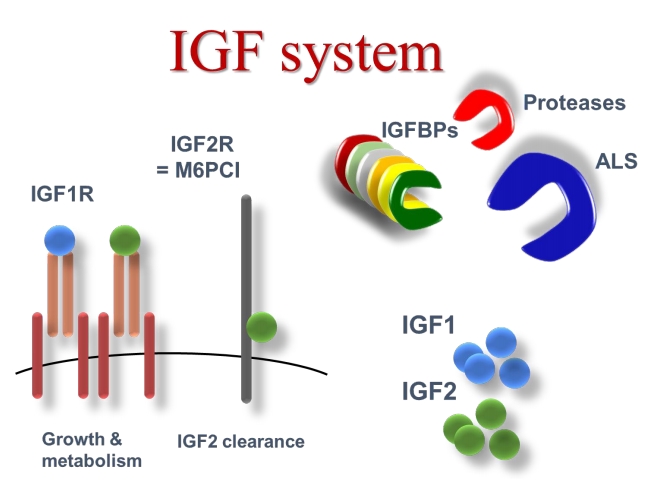
GH is the main regulator of hepatic IGF-I production. IGF-I, in turn, modulates GH release in a negative feedback loop and mediates the growth promoting action of GH in cartilage [2]. IGF-I and IGF-II comprise a family of peptides structurally related to insulin that promote cell growth through interaction with specific high affinity receptors [3]. Circulating IGFs are synthesized primarily in the liver and exert an endocrine function. There is good correlation between growth and IGF-I levels [4]. and data from IGF-I transgenic mice have definitively proved the growth-promoting action of IGF-I in vivo [5]. IGF-I is also produced by most, if not all, tissues and acts in an autocrine-paracrine mode [4]. Both serum and tissue IGFs are bound to specific binding proteins (IGFBPs) and six structurally different IGFBPs (termed IGFBPs 1–6) have been identified and sequenced [6,7]. IGFBPs function not only as carriers but also modulate the release of IGFs to the tissues [7,8]. IGFs circulate in three different forms: unbound (free), in binary complexes with IGF-binding protein (IGFBP) 1 to 6, and in ternary complexes containing an IGFBP and the approximately 85-kDa glycoprotein known as the acid-labile subunit (ALS) [9]. The circulating half-lives of IGF-I and IGF-II are reported to be about 10 minutes in the free form, less than 30 minutes in binary complexes, and 12–15 hours in the ternary complexed form [10]. IGFBP-3 has been extensively documented as being unique among the IGFBPs in its ability to form ternary complexes with the IGFs and ALS [11]. These complexes form a circulating reservoir of IGFBP-3 and IGFs as, unlike free and binary complexed IGFs, they do not cross the capillary barrier [12]. IGFBP-5, like IGFBP-3, is able to form heterotrimers by combining with IGF-I or IGF-II and ALS [13].
IGFBPs, in turn, are under control of specific proteases which, by fragmenting the IGFBP molecule, regulate the affinity and, consequently, the release of IGFs from IGFBPs [14]. In peripheral tissues, IGFs bind to specific cell surface receptors, which mediate their mitogenic and anti-apoptotic effects [3,11]. The IGF-I receptor is the main mediator of the biological actions of both IGF-I and IGF-II. The insulin receptor mediates some of the IGF-II cellular effects [15]. The IGF-II/M-6-P receptor does not play any role in IGF signal transduction but is responsible for clearing, and thereby reducing, the circulating levels of IGF-II [16]. Fig. 1 shows the IGF system.
Growth hormone/IGF-I axis and cancer risk in experimental models
The GH/IGF-I axis activation seems to play a role in different types of cancer. Prostate, breast, endometrial and colorectal neoplasms express both GH and GH receptor and other tumors express GH releasing hormone [1]. Furthermore, the overexpression of GH increases proliferation and promotes survival of different cancer cell lines [17-19].
The IGFs are recognized as important growth factors in many tumor cell types [20] and virtually all human cancer cells express IGF receptors [21] and produce IGFs [22], IGFBPs [23], and IGFBP proteases [24]. In other words, many tumors locally create a specific IGF system that sustains their own growth and dissemination. The type I IGF receptor is actively involved in the control of cell growth and differentiation, and mutations of this receptor inhibit the development of tumors in mice [25]. Finally, there is increasing evidence that IGFs are also able to stimulate cell motility. Many types of cells, such as endothelial cells, keratinocytes, osteoblasts, rhabdomyosarcoma cells, epithelial cells, trophoblasts, melanoma cells, breast cancer cells, smooth muscle cells, carcinoma cells and neuroblastoma cells, migrate toward a source of IGFs or display increased motility in the presence of these factors [26,27]. Taken together, these findings seem to confirm the original hypothesis of Sporn and Roberts [28] who first suggested that growth factors play a pivotal role in cancer development and growth.
The potential role of the GH-IGF axis in cancer development has also been confirmed in different animal models. The animals with reduced production/action of GH and IGF-I are resistant to carcinogenesis [29-31]. On the contrary, mice transgenic for human GH display increased rates of tumors [32].
Growth hormone/IGF-I axis and cancer risk in humans
Whilst the strength of evidence for a relationship between GH/IGF-I axis and cancer risk is high in both cellular and animal models, data in humans are scarce and conflicting.
Epidemiological studies have shown an association between elevated circulating levels of IGF-I and an increased risk of developing certain cancers such as prostate, breast and colorectal neoplasms. (Box 1) [33-37] The association between GH-IGF and carcinogenesis is also suggested by the observation that patients suffering from acromegaly, an endocrine disorder characterized by sustained hypersecretion of GH and consequent increased levels of IGF-I, have a higher risk of developing colorectal and thyroid cancer [38-42]. On the other side, patients with either primary or secondary IGF-I deficiency seem to be protected from developing malignancies [43-45].
Overall these data indicate an association, not a causal relationship. Genetic predisposition as well as common nutritional and environmental factors may in fact account for these associations.
Association of GH therapy in childhood with cancer morbidity and mortality
The first report suggesting a potential link between GH treatment and malignancy dates back to the '80s when leukemia risk was associated with the use of GH [46]. Further detailed analysis of these cases revealed that these patients had concomitant conditions predisposing them to cancer independently of GH therapy. The risk of leukemia was not increased in children treated with GH in the United States according to the National Cooperative Growth study initiated in 1985 [47]. In 2002, a long-term study of 1,848 patients treated with human pituitary GH during childhood and early adulthood showed an increased risk of colorectal cancer and Hodgkin lymphoma (HL) [48]. However, it has to be pointed out that the absolute number of recorded deaths for colorectal cancer was 2 against an expected number of 0.18 and the absolute number of cases with colorectal cancer and HL was 2 against an expected number of 0.13. Therefore, in spite of the statistical significance, the absolute number of cases was extremely low. Nevertheless, although based on small numbers, the observed risk of neoplasms raised some concern and paved the way for further long-term observational studies.
In 2014 we performed a systematic review and meta-analysis of published studies reporting data on long-term impact of r-hGH therapy on the risk of cancer mortality and morbidity in patients treated during childhood. Three studies reporting the standard mortality ratio (SMR) [49-51], three reporting the standard incidence ratio (SIR) [47,52,53] and 3 reporting the overall relative risk (RR) of second neoplasms [54-56], were analyzed. The results showed that malignancy SMR was not significantly increased whereas overall cancer SIR (2.74; 95% confidence interval [CI], 1.18–4.41) and the RR for second neoplasms (1.99; 95% CI, 1.28–3.08) were significantly increased [57]. However, all the studies analyzed were affected by a number of confounders and biases such as: (1) the heterogeneity of the study populations comprising both adult and pediatric cohorts and including patients with different diagnoses; (2) the limited sample size; (3) the low event rate; (4) the lack of an untreated control group; (5) the lack of key data such as familial predisposition to diseases and exposure to environmental hazards; (6) the lack of local mortality and morbidity indices; (7) the lack of information on dose and treatment duration.
In 2012, two independent studies on long-term mortality in patients treated with r-hGH during childhood, published in the same issue of the same journal [51,58], reported opposite results. The French study showed a significant increase in mortality for bone tumors in a cohort of about 6,500 young adult subjects treated with r-hGH during childhood for the indications of isolated GH deficiency (IGHD), short stature associated with small for gestational age (SGA), or idiopathic short stature (ISS) [51]. The other study involving cohorts from Sweden, The Netherlands, and Belgium, reported not a single case of death from cancer in about 2,500 subjects with the same diagnostic categories [58].
A more recent Swedish study applied a novel mortality model using continuous hazard-functions adjusting for birth-characteristics, gender, age-intervals, and calendar-year to estimate SMR in a population of 3,847 patients diagnosed with IGHD (n=1,890), ISS (n=975), and SGA (n=982). Compared with the general Swedish population, the ratio of observed/expected deaths (21/21.99) was not increased in childhood r-hGH-treated IGHD, ISS, and SGA-patients after adjusting for birth-characteristics [59].
In the GeNeSIS (Genetics and Neuroendocrinology of Short Stature International Study) prospective, multinational, observational study sponsored by Eli Lilly and conducted on 9,505 GH-treated patients with different diagnoses followed for at least 4 years, no significant increase in cancer mortality was observed in IGHD, ISS, and SGA patients [60]. These findings have recently been confirmed in a further analysis conducted on over 20,000 GeNeSIS patients [61].
A position paper delivered by the international scientific societies involved in the use of r-hGH in both children and adults, stated that the available evidence in children does not indicate either an increased risk of new primary cancers or an increased risk of recurrence of primary cancer in GH recipients [62]. The risk of second neoplasms in GH-treated survivors of pediatric cancers seems to be increased but declining with longer follow-up [55,62-64].
The Safety and Appropriateness of Growth Hormone Treatments in Europe study
Most observational studies conducted in patients treated with r-hGH have been based on pharmaceutical databases and have reported short-term follow-up results. The SAGhE (Safety and Appropriateness of Growth Hormone Treatments in Europe) study was conceived to provide a large-scale European collaborative cohort study of r-hGH-treated patients with long-term follow-up for cancer incidence and mortality, independently of pharmaceutical companies.
The SAGhE study recruited cohorts of patients from 8 European countries treated in childhood with r-hGH. The study population consisted of 24,232 patients and represents the largest and longest follow-up cohort of GH-treated patients with follow-up and analysis, independent of industry [65].
The SAGhE population was classified according to the initial diagnoses into three different classes of cancer risk: (1) low risk: isolated growth failure, including IGHD, ISS and SGA; (2) high risk: cancer, including patients with previous history of cancer; (3) intermediate risk: nonisolated growth failure and non-cancer patients, including all the other patients such as multiple pituitary deficiencies, Turner syndrome, Noonan syndrome and bone dysplasias. The cohort for cancer related mortality risk comprised 23,984 patients and for cancer incidence 10,406 patients. The average length of follow-up for mortality was 16.5 years per patient, and for cancer incidence 14.8 years per patient. The mean age at the end of follow-up was 27.1 years for the cancer mortality analysis and 25.8 years for the incidence analysis [66].
In the low risk (isolated growth failure) group, both mortality and morbidity for cancer was not increased. SMR and SIR for many types of cancers were significantly increased in GH-treated patients of the high-risk group. In the intermediate risk group, the incidence of bone and bladder cancers was significantly raised in GH-treated patients. Cancer risk was unrelated to the duration or cumulative dose of r-hGH treatment, but for patients treated after previous cancer, cancer mortality risk increased significantly with increasing daily r-hGH dose. Finally, the HL incidence increased significantly with longer follow-up (P trend=0.001 for patients overall and 0.002 for patients without previous cancer) [66].
Although the SAGhE study is more accurate and informative than most previous observational studies, it nonetheless shares a number of biases and confounders that affect the interpretation of results (Box 2) and, although more informative than most previous observational studies, even the SAGhE has intrinsic weaknesses that limit its epidemiological and clinical value (Box 3).
Conclusion
The available evidence does not indicate an increased risk of cancer within the length of follow-up currently available especially for low risk children (IGHD, ISS, and SGA). However, it has to be pointed out that data from the French cohort of the SAGhE population indicate an increased risk for bone tumor mortality and morbidity even in the low risk cohorts [51,67]. The available data suggest that r-hGH treatment does not substantially increase leukemia risk in patients without prior high risk, but it is unclear whether the risk is increased in high-risk individuals [46,47,66]. Increased risk of mortality for HL in children treated with pituitary derived GH was reported [48]. This finding was not confirmed in the SAGhE study though a significant trend with longer follow-up (although no trend with GH dose) was observed [66].
Although overall the results from the observational studies are reassuring, a number of biases, confounders and weaknesses limit the value and interpretation of all data reported so far. The use of r-hGH as replacement treatment to accelerate growth and improve adult height appears fully justified in conditions where therapy replaces an unequivocal deficiency. The pharmacological use of r-hGH in short children with sufficient GH secretion raises the issue of benefit against potential adverse effects and cost to the healthcare system. The establishment and follow-up of lifespan cohorts consisting of patients treated with GH during childhood, adolescence, and adult life would be needed to properly address the issue of long-term GH safety. Such cohorts should be characterized by: (1) adequate sample size and statistical power; (2) careful characterization of patients relative to the underlying disorder (including etiology, severity, genetic syndromes, comorbidities, and response to treatment), as well as socio-demographics; (3) accurate documentation of GH treatment and response; (4) measurement of IGF-I concentrations; (5) comprehensive long-term surveillance, including documentation of all adverse outcomes; and (6) last, but not least, an appropriate control group [68,69].
Box 1. Key background points
• GH treatment causes exposure of tissues to increased GH and IGF-I levels.
• GH and IGF-I have the potential to promote tumor growth and progression (in cellular and animal models) but do not lead to abnormal differentiation of cells.
• Acromegaly, a condition characterized by long-standing excess GH secretion, is associated with increased risk of colorectal and thyroid cancers.
• Cumulative evidence from epidemiological studies supports an association between elevated circulating levels of IGF-I and an increased risk of certain cancers.
Box 2. Biases and confounders in all available studies
• No comparison data for untreated patients.
• Insufficient duration and completeness of follow-up.
• Multicenter study (missing data, unidentified confounders).
• Population heterogeneity (age, diagnosis).
• Limited sample size.
• Low event rate.
• Lack of key data such as familial predisposition to certain diseases, exposure to environmental hazards, lack of local mortality and morbidity indices.
Box 3. Biases and confounders in all available studies
• No information on GH treatment beyond pediatric ages, so treatment duration for some patients may have been underestimated with consequent dilution of any true effect of duration on cancer risk.
• Aggregation of data from eight (SAGhE) countries adds to the complexity of heterogeneity in patient mix and treatments.
• No information on IGF-I levels.
• Follow-up included few person-years beyond age 35, and hence had limited power for cancers prevalent at and past middle age.
• It is important to distinguish SMR from other metrics such as absolute risk and number-needed-to- harm, with the latter being more relevant to counselling patients and families about risk.
Notes
Conflict of interest No potential conflict of interest relevant to this article was reported.