Screening and management of thyroid dysfunction in preterm infants
Article information
Abstract
Preterm infants can suffer various thyroid dysfunctions associated with developmental immaturity of the hypothalamic-pituitary-thyroid axis, postnatal illness, medications, or iodine supply. The incidence of thyroid dysfunction among preterm infants is higher than that among term infants and has been increasing with improvement in the survival of preterm infants. Hypothyroxinemia is frequently observed during the first week of life in extreme preterm neonates, and the incidence of delayed thyrotropin elevation is high at the age of 2–6 weeks. Although the necessity of routine rescreening remains controversial, recent guidelines on screening for congenital hypothyroidism have recommended rescreening of all preterm neonates. Thyroid hormone replacement is recommended for persistent thyrotropin elevation with or without hypothyroxinemia. Hypothyroxinemia without thyrotropin elevation does not require treatment, and some potential risks of levothyroxine supplementation have been reported. Although most thyroid dysfunctions are transient, careful follow-up after discontinuation of levothyroxine is considered so as to avoid missing persistent hypothyroidism.
Introduction
Thyroid hormone is essential for the growth and development of infants. A profound deficiency of thyroid hormone during the early period of life is the leading cause for mental retardation, which is preventable with proper treatment. The importance of early diagnosis and treatment of congenital hypothyroidism, which was highlighted through the first neonatal screening program for congenital hypothyroidism, was established in the mid-1970s in Quebec [1]; such a screening program is now conducted in many countries. In South Korea, a government-led neonatal screening program began in the early 1990s, and a nation-wide screening program was established in the mid-2000s [2]. Although there is considerable amount of historic data regarding screening programs for congenital hypothyroidism, the standard methods still remain controversial [3].
The incidence of premature birth is increasing, and advances in neonatal care have improved the survival of preterm neonates who present thyroid dysfunction more frequently than term neonates. Preterm neonates are considered as a special category of neonates who are at a risk of congenital hypothyroidism [4,5]. In 2014, the European Society for Pediatric Endocrinology (ESPE), on behalf of all pediatric endocrinologist societies worldwide, published updated guidelines on congenital hypothyroidism and recommended rescreening for congenital hypothyroidism among all preterm neonates [5]. Other recent guidelines, such as those published in the Japanese Society for Pediatric Endocrinology and Indian Society for Pediatric and Adolescent Endocrinology, have also specified methods of screening for preterm infants [6,7]. However, the optimal management of thyroid dysfunction among preterm neonates remains unclear.
This article reviews the characteristics of thyroid function in preterm infants, compares recent guidelines regarding screening for congenital hypothyroidism among preterm infants, and determines the challenges regarding thyroid dysfunction in preterm infants that need to be addressed.
The characteristics of thyroid function in preterm infants
The postnatal thyrotropin (TSH) surge is blunted and serum thyroxine (T4) concentration is frequently low at 1–2 weeks of postnatal life in preterm neonates [8-10]. In addition, delayed TSH elevation from normal TSH levels are observed in the initial tests among some preterm infants [10,11].
1. Transient hypothyroxinemia of prematurity
Transient hypothyroxinemia of prematurity (THOP) is characterized by transiently low levels of circulating thyroid hormones along with normal TSH levels [10]. The depth of the nadir and length of time before the recovery of THOP are related to gestational age (GA). THOP usually resolves within 2–3 weeks accompanied with progressive maturation of the hypothalamic-pituitary-thyroid (H-P-T) axis [10]. The prevalence of THOP has been reported to be 35%–85% among very preterm infants [12]. Blunted TSH surge after birth, decreased hepatic production of T4-binding globulin, reduced iodine storage, dopamine administration, high-dose steroid therapy, exchange transfusion, and undernutrition contribute to THOP development [9-13].
Although transiently low levels of thyroid hormones are associated with high incidence rates of cerebral palsy and cognitive impairment in preterm infants [14], previous studies have not demonstrated any clear advantages of thyroid hormone replacement [15-18]. The current recommendation is to administer thyroid hormone alone to infants with THOP if the condition is characterized by elevated TSH levels [6,19].
When a preterm neonate presents low T4 levels along with normal or low TSH levels, a differential diagnosis for central hypothyroidism should be made in certain clinical situations. A thyrotropin-releasing hormone (TRH) stimulation test might differentiate them because the response of infants with THOP to TRH stimulation test is not different to that of euthyroid infants [20].
2. Delayed TSH elevation
Transient TSH elevation for several weeks after birth is predominant among preterm infants, low birth weight (LBW) infants, and very LBW (VLBW) infants. This phenomenon is called "delayed TSH elevation" or "atypical hypothyroidism." It usually occurs at 2–6 weeks of age and most cases resolve at 6–10 weeks of age [11].
The etiology of delayed TSH elevation remains unclear. An elevated TSH concentration may reflect true primary hypothyroidism or the recovery of illness-induced suppression of the H-P-T axis. Iodine deficiency or excess iodine levels are associated with the development of delayed TSH elevation in some preterm infants [10,11,21].
According to an Italian study, delayed TSH elevation reportedly affected 0.2% of LBW infants [22]. In South Korea, delayed TSH elevation requiring levothyroxine replacement was reported in 7.6% of infants with a GA of <32 weeks and in 8.9% of infants with a birth weight of <1,500 g [23,24]. Delayed TSH elevation is more frequent in infants with a lower GA, lower birth weight, or a serious illness. In a study performed in New England, the incidence of delayed TSH elevation was 1 in 58 for extremely low-birth-weight infants, 1 in 95 for VLBW infants, and 1 in 30,329 for LBW infants [25].
Levothyroxine supplementation is usually recommended for TSH elevation and/or low T4 level [3,19,26].
The main factors that affect thyroid function in preterm infants
Thyroid function in preterm infants reflects the relative immaturity of the H-P-T axis found in the fetuses with a comparable GA. Environmental factors also influence thyroid function in preterm infants. Table 1 summarizes the factors that affect thyroid function in preterm infants.
1. Developmental immaturity
During fetal life, circulating levels of T4 and triiodothyronine (T3) are low, whereas those of the inactive metabolites, reverse T3 (rT3) and T3 sulfate, are high. This is a consequence of both the immaturity of the H-P-T axis and coordinated adjustments in the deiodinase system. The maturation of the H-P-T axis continues until late gestation [9,10]. The activity of type 1 iodothyronine deiodinase (D1) is low, and the levels of type 2 deiodinase (D2) and type 3 deiodinase (D3) are high throughout gestation [25]. The near-term increase in fetal cortisol and T4 levels induces tissue D1 activities and reduces the levels of D2 and sulfotransferase. This change is limited prior to 30 weeks, which results in low serum levels of T3 and relatively high levels of inactive rT3 and sulfated iodothyronines in preterm neonates. The increasing levels of free T4 stimulate hepatic D1 activity in preterm infants, but this phenomenon requires several weeks [10,27,28]. The serum concentration of thyroxine-binding globulin, prealbumin, and albumin are low in preterm infants, which is associated with low serum T4 level [13,29].
The thyroid gland of preterm neonates is smaller than that of term neonates. The mean value of thyroid weight in preterm neonates of GA 24–32 weeks was reported to be 0.44 g vs. 1.79 g in term neonates [30]. According to the size of the thyroid gland, thyroid hormone synthesis and storage capacity as well as iodine content in preterm neonates are lower than those in term neonates. A small thyroid gland as well as low thyroid hormone and iodine reservoirs are associated with the development of thyroid dysfunction when thyroid hormone requirement rapidly changes or iodine imbalance occurs.
2. Environmental factors
Various medications, particularly dopamine and steroids, affect thyroid function (Table 2) Neonatal morbidity, such as respiratory distress syndrome and malnutrition, also affects thyroid function in preterm infants [10,23,31].
Preterm neonates have a narrow range for safe iodine intake. Studies conducted on healthy preterm and full-term newborns led us to believe that the iodine intake required to maintain a positive balance is 15 μg/kg per day among full-term newborns and 30–60 μg/kg per day among preterm neonates [32]. Preterm neonates are more vulnerable to thyroid dysfunction caused by iodine deficiency than term neonates because of a much lower capacity of the thyroid gland of preterm neonates to retain iodine [10]. Studies on iodine balance have shown that most sick or extreme-preterm neonates have iodine deficiency [33-35]. This is particularly significant in iodine-deficient areas worldwide. Meanwhile, preterm neonates are vulnerable to thyroid suppression caused by excess iodine levels [36-38]. Impaired iodine organification in the human fetus can increase the Wolff-Chaikoff effect, and escape from the Wolff-Chaikoff effect seems to appear at a GA of >35 weeks among preterm infants [39]. Neonatal exposure to iodine-containing disinfectants causes thyroid dysfunction among infants born at <32 weeks [40]. Maternal iodine intake is related to iodine content in breast milk [37,41]. Excessive iodine in breast milk is associated with subclinical hypothyroidism among preterm infants [38].
Enteral iodine intake at 30–100 μg/kg per day is widely accepted for preterm infants, whereas that for term infants ranges from 15 to 150 μg/kg per day [33,42,43]. The recommended parenteral intake of iodine is 1 μg/kg per day, which is far below the requirement for preterm neonates [44-46].
Controversies regarding congenital hypothyroidism screening
There are 2 major screening strategies targeted toward the detection of congenital hypothyroidism: primary T4/backup TSH method and primary TSH method. A primary T4/backup TSH program is favorable for the detection of hypothyroxinemia, although this method is unable to detect subclinical hypothyroidism or normal T4 with delayed TSH elevation. Meanwhile, the primary TSH program overlooks the detection of hypothyroxinemia without TSH elevation, and a single TSH program will also overlook delayed TSH elevation. The primary T4/backup TSH program is favored in North America, and the primary TSH program is favored in most parts of Europe and Asia including Korea. According to the ESPE guidelines, primary TSH screening is the most sensitive test for detecting primary congenital hypothyroidism [5].
Most programs collect one blood sample within the first week of life. However, the reliability of a single screening is particularly low among preterm neonates than among term neonates because preterm neonates frequently present with transient hypothyroxinemia and lack a predictable pattern of TSH secretion. Thus, recent screening guidelines for congenital hypothyroidism have recommended routine rescreening of preterm neonates (Table 3) [4-7,47].
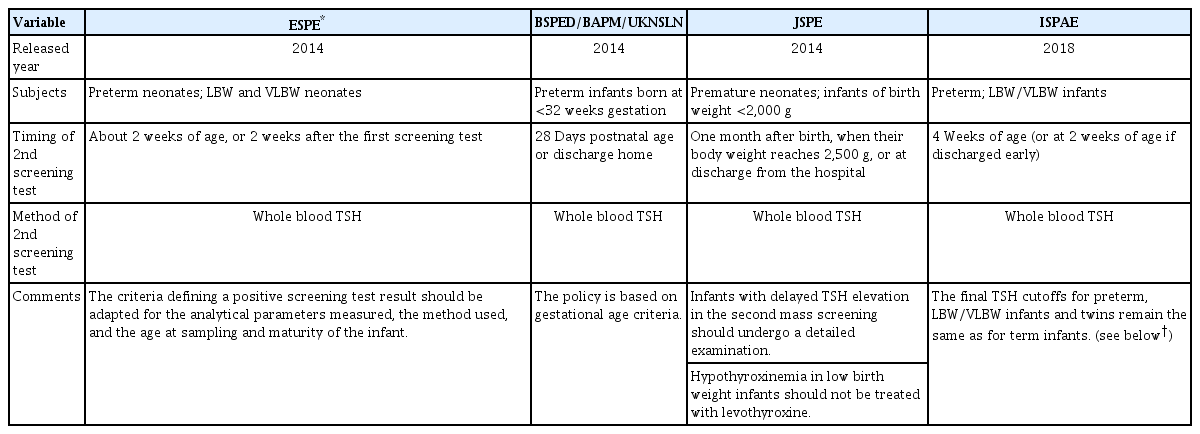
Several guidelines on screening for congenital hypothyroidism in preterm or low birth weight infants
However, not all centers have adopted the routine rescreening strategy based on the following considerations: (1) VLBW newborns with permanent primary hypothyroidism can mount an appropriate TSH response, (2) most of the thyroid dysfunctions are transient problems, and (3) the benefits of treatment for mild thyroid dysfunction remain unproven [25,48]. Moreover, rescreening of all preterm neonates adds to the stress of frequent samplings and a high economic burden.
Therefore, stratified screening according to the GA or birth weight or initial screening result warrants investigation. LaFranchi [49] suggested a routine repeated screening test for neonates having a birth weight of <1,500 g or GA of <32 weeks. Korada et al. [50] reported that in case of an initial blood TSH level of <6 mU/L, no thyroid abnormalities requiring treatment were noted; thus, a second sample might not be necessary for a screening threshold of 6 mU/L. Nonetheless, it is challenging to determine the categories that do not require repeated screening because the TSH values of preterm neonates are usually unpredictable.
Rescreening is mostly performed at the age of 2–4 weeks when a delayed TSH elevation is prominent. A few programs have recommended repetition of the rescreening procedure at a 1-month interval because extremely premature infants can subsequently develop hypothyroidism. However, the longterm outcome data are limited for infants who had normal first or second screening results but subsequently experienced an elevation in TSH levels [25].
Issues on thyroid hormone replacement
The interpretation of screening result is another problem. Some screening programs have suggested similar cutoffs for TSH or T4 among term and preterm infants [7], while others have recommended use of normal values for thyroid hormones and TSH according to GA and postnatal age [27,49,51]. In 2006, the American Academy of Pediatrics suggested a reference TSH range of 1.7–9.1 mU/L for preterm infants at the age of 2–6 weeks [4]. However, this does not mean that TSH levels above the reference value adversely affect the health of infants. Woo et al. [25] reported that preterm infants with an elevated TSH level of <50 mIU/L (mean, 21 mU/L) were not treated, and the growth and neurological outcomes of infants with delayed TSH elevation were similar to those of control infants at 18-month follow-up. Considering that the major purpose of neonatal screening for congenital hypothyroidism is the detection of neonate with severe hypothyroidism who would develop disabilities if remained untreated, the initiation of thyroid hormone replacement in all infants who present an abnormal thyroid hormone profile might be unnecessary. Levothyroxine supplementation for "persistent or profound TSH elevation and/or low T4 level" is usually recommended, although the criteria for treating these conditions have not been clearly established [3]. The screening methods used in several studies on thyroid function of preterm infants in Korea are summarized in Table 4.
Administration of levothyroxine to preterm infants in Japan has been suggested to cause late-onset circulatory collapse [52,53]. Kawa et al. [53] reported that the administration of levothyroxine was associated with an increased risk of late-onset circulatory collapse in VLBW infants, and that the first 2 weeks after the initiation of supplementation could be a high-risk period. However, the causal relationship was not proven in other studies [54-56]. Although there is incomplete evidence, but Japanese guidelines have recommended that hypothyroxinemia in LBW infants should not be treated with levothyroxine.
The starting dose for infants with delayed TSH elevation is often lower than the dose used to treat typical congenital primary hypothyroidism (8–10 μg/kg per day vs. 10–15 μg/kg per day), while the dose for preterm neonates with obvious primary hypothyroidism is similar to that for term neonates [49].
Re-evaluation of thyroid function is recommended for all preterm infants with normally located gland or for cases in which no etiological diagnostic assessment was carried out during early infancy [5]. Re-evaluation is possible before 3 years of age in preterm infants. Lim et al. [57] and Jung et al. [58] reported that discontinuation of thyroxine supplementation is possible at the age of ≤2 when low doses of levothyroxine administration could be successfully maintained.
Some infants present persistent thyroid dysfunction after discontinuation of thyroid hormone replacement, although most thyroid dysfunctions among preterm infants are transient. Vigone et al. [59] reported that among 23 preterm infants diagnosed with congenital hypothyroidism by carrying out the screening procedure, 12 infants were diagnosed either with permanent hypothyroidism or persistent hyperthyrotropinemia. Most infants with permanent hypothyroidism were associated with twin birth, assisted birth, lower GA, and severe postnatal complications-related to prematurity. Thus, preterm infants, particularly those having the abovementioned features, should be carefully followed up by thyroid function testing.
Conclusions
Preterm infants have a higher incidence of thyroid dysfunction that includes a unique form of hypothyroidism. While several guidelines have been published on screening for congenital hypothyroidism over the recent decades, optimal management and long-term outcomes of thyroid dysfunction in preterm infants remain unclear. Thyroid function in preterm infants is affected by various factors including immaturity of the H-P-T axis, postnatal medical condition, and regional iodine supply; thus, advanced clinical guidelines that can take these conditions into account are warranted.
Notes
Conflict of interest: No potential conflict of interest relevant to this article was reported.



