Multiple daily injection of insulin regimen for a 10-month-old infant with type 1 diabetes mellitus and diabetic ketoacidosis
Article information
Abstract
The incidence of type 1 diabetes is increasing worldwide, and the greatest increase has been observed in very young children under 4 years of age. A case of infantile diabetic ketoacidosis in a 10-month-old male infant was encountered by these authors. The infant's fasting glucose level was 490 mg/dL, his PH was 7.13, his pCO2 was 15 mmHg, and his bicarbonate level was 5.0 mmol/L. The glycosylated hemoglobin level had increased to 9.4%. Ketonuria and glucosuria were detected in the urinalysis. The fasting C-peptide and insulin levels had decreased. The infant was positive for anti-insulin and antiglutamic acid decarboxylase antibodies. Immediately after the infant's admission, fluid therapy and intravenous insulin infusion therapy were started. On the second day of the infant's hospitalization and after fluid therapy, he recovered from his lethargic condition, and his general condition improved. Feeding was started on the third day, and he was fed a formula 5 to 7 times a day and ate rice, vegetables, and lean meat. Due to the frequent feeding, the frequency of rapid-acting insulin injection was increased from 3 times before feeding to 5 times, adjusted according to the feeding frequency. The total dose of insulin that was injected was 0.8–1.1 IU/kg/day, and the infant was discharged on the 12th day of his hospitalization. The case is presented herein with a brief review of the relevant literature.
Introduction
Diabetic ketoacidosis (DKA) is a complex metabolic state of hyperglycemia, ketosis, and acidosis. It is the leading cause of mortality in childhood diabetes mellitus (DM). The younger the child is, the more difficult it is to obtain the classical history of polyuria, polydipsia, and weight loss12). In infants as well, a frequent feeding schedule makes it challenging to develop a management plan that avoids episodes of hypoglycemia but provides sufficient glycemic control. In addition, the stress caused by implementing the daily care plan can have an adverse effect on parents34). Thus, the rise in the incidence of type 1 DM in very young children poses a challenge to pediatric practitioners. Here, a case of a 10-month-old male infant with DKA due to type 1 DM who had anti-insulin and anti-glutamic acid decarboxylase (GAD) antibodies is described, along with how the patient was managed according to his feeding schedule.
Case report
A 10-month-old male infant was transferred to the authors' hospital due to a high blood glucose level (710 mg/dL) along with a markedly dehydrated and lethargic condition. He had had fever for 3 days, and a furuncle on the left side of the scalp. He had lost 12% of his body weight over 5 months. He also had polydypsia (500 mL water intake 1 to 2 times per day) and polyuria. His diapers were usually changed 6 times a day, but the frequency of diaper changing was increased to 10 times a day 3 days before he was brought to the authors' hospital. His birth weight was 2,780 g (10th–25th percentile for his age), and he was born after 38 weeks of gestation and had no previous illness history. He had been fed only formula milk, and weaning foods were about to be introduced.
At admission, the infant's body weight was 10 kg (50th–75th percentile for his age), and his height was 74 cm (25th–50th percentile for his age). In the physical examination, his temperature was 37.2℃, his heart rate was 149 beats/min, his respiratory rate was 52 breaths/min, and his blood pressure was 105/75 mmHg. In the laboratory tests, his fasting glucose level was 490 mg/dL. The initial arterial blood gas analysis revealed the following parameters: pH 7.13 (normal range, 7.35–7.45), pCO2 15 mmHg (normal range, 35–48 mmHg), and bicarbonate 5.0 mmol/L (normal range, 21–28 mmol/L). The glycosylated hemoglobin (HbA1c) level had increased to 9.4% (normal range, 5.0%–7.0%). Ketonuria and glucosuria were detected in the urinalysis. The fasting C-peptide level was 0.19 ng/mL (normal range, 0.48–3.3 ng/mL), and the fasting insulin level was 0.82 IU/mL (normal range, 2.1–22.0 IU/mL). He was positive for anti-insulin and anti-GAD antibodies (anti-insulin antibody, 64.77%; normal range, <7%; anti-GAD antibody, 6.6 IU/mL; normal range, ≤1.0 IU/mL) and was negative for antiislet antibody. Based on the laboratory tests, the infant was diagnosed as having moderate to severe DKA due to type 1 DM.
Immediately after admission, fluid therapy and intravenous insulin infusion therapy were started. The peripheral circulation was restored with normal saline with 20 mEq/L potassium acetate and 20 mEq/L potassium phosphate. The fluid mixing was modulated based on the patient's electrolyte level. Insulin was administered at 0.1 IU/kg/hr, and the rate of insulin infusion was reduced according to the infant's glucose level. On the second day of hospitalization, the infant recovered from his lethargic condition, and his general condition improved. His temperature normalized on the third day after the injection of intravenous antibiotics. Feeding was started on the third day, and multiple subcutaneous insulin injections were started. He was fed formula milk 5 to 7 times a day and ate rice, vegetables, and lean meat. Due to the frequent feeding, the infant's glucose level fluctuated between 100 and 600 mg/dL. Rapid-acting Novorapid Flexpen (Norvo Nordisk Pharma, South, Korea) and long-acting Levemir Flexpen (Norvo Nordisk Pharma, South, Korea) were used. Initially, 0.1–0.2 IU/kg Novorapid Flexpen was injected 3 times before meals, usually at 7 to 8 o'clock in the morning, 1 to 2 o'clock in the afternoon, and 7–8 o'clock in the evening. As for Levemir Flexpen, 0.4 IU/kg of it was injected at about 11 o'clock, before bedtime. The infant's glucose level still fluctuated, however, based on the feeding; hence, the frequency of rapid-acting insulin injection was increased from 3 times before feeding to 5 times, adjusted according to the feeding frequency (Fig. 1). The total dose of insulin that was initially injected was 0.8–1.0 IU/kg/day. When the frequency of insulin injection was increased, the total dose was 0.8–1.1 IU/kg/day. The infant was discharged on the 12th day of hospitalization, when his blood glucose level stabilized at 157–280 mg/dL.
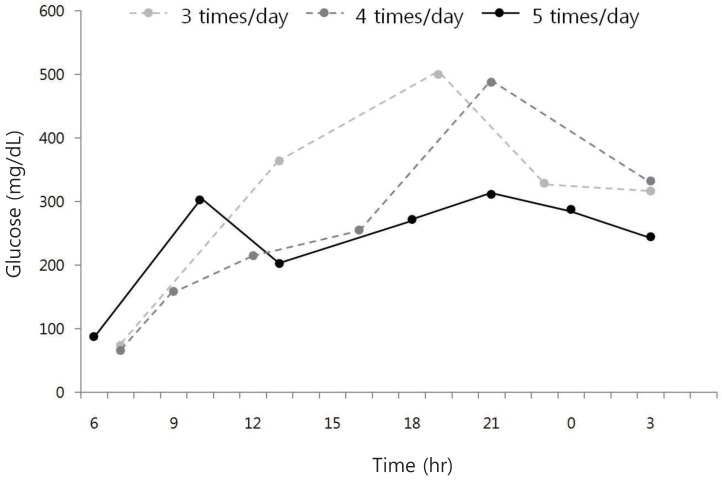
Comparison of glucose level according to the frequency of rapid acting insulin injections in an infant with type 1 diabetes mellitus. The glucose level was more stable when more frequent injections of rapid acting insulin were administered based on the infant's feeding schedule.
The infant's current age is 14 months, and he weighs 11.7 kg. He experienced episodes of hypoglycemia due to the honeymoon period; hence, the dose of insulin injection was reduced from 0.9 IU/kg/day to 0.41–0.52 IU/kg/day. His recent HbA1c level is 8.3%.
Discussion
As mentioned earlier, the rising incidence of type 1 DM is a global phenomenon15). In a cohort of pediatric patients who had new-onset DM with DKA, the percentage of children under 5 years of age with DKA as the initial diagnosis was 36%, compared to 16% of adolescents older than 14 years of age1). Also, it is known that breastfeeding may lower the risk of type 1 DM either directly or by delaying the exposure to cow's milk protein6). The patient in the case presented herein was fed only formula milk.
Autoimmunity precedes the onset of clinical type 1 DM; hence, autoimmune responses may be useful markers for disease prediction. The number of positive autoantibodies can help estimate the risk of developing type 1 DM: low risk (single autoantibody), moderate risk (2 autoantibodies), or high risk (>2 autoantibodies) over a 5-year period6). The patient in the case presented herein had two positive autoantibodies.
After diagnosis, the management of DM in infants or toddlers is very different from that in older children because of their peculiar features and clinical characteristics. Infants and toddlers have a wide range of serum glucose levels due to their variable daily activities and diet, and also due to their rapid growth spurts and frequent illness. They are very sensitive to tiny doses of insulin, and this adds to the trouble with regard to glucose control13). The authors also had difficulty controlling the infant's glucose level. His glucose level fluctuated based on the feeding, and it was difficult to adjust the insulin injection dose and frequency.
Generally, caloric and glucose restriction should be avoided, and the patient should be fed regularly with complex-carbohydrate-based meals and snacks to reduce the risk of hypoglycemia, with continuous feeding to limit the potential hyperglycemia37). The patient in the case presented herein was in the growing phase, and weaning food was about to be introduced; hence, the Nutrition Support Team was consulted, and a feeding schedule was designed. The patient was regularly fed, ensuring that he had sufficient caloric intake.
In the past, infants and toddlers with diabetes were treated with 1 to 2 daily injections of 10% or 20% rapid/intermediate insulin mixture. More recently, due to the difficulties with the fluctuations of the serum glucose level at this age, the use of basal-bolus therapy with long-acting insulin analogs and multiple premeal injections of rapid-acting analogs are increasing78).
In infants receiving parenteral nutrition, the total daily dose of insulin should be given as basal infusion37). With more than 6 breastfeeds per day, high basal insulin substitution with very low mealtime boluses, which allows for stable blood glucose levels, is recommended. In the case of intermittent oral feeding, the increased insulin requirements associated to meals require increased doses of rapid-acting insulin, and the basal insulin has to be reduced accordingly7). As the food intake of infants is frequently unpredictable, immediate postprandial insulin administration may be considered37). In the case presented herein, multiple insulin injections with long-acting insulin analog were started. The patient also had an unstable glucose level with the feeding schedule; hence, the frequency of injection of rapid-acting insulin analog adjusted according to the feeding time was increased. In the case presented herein, the total insulin injection dose was observed to be almost the same; hence, it is believed that the frequency of insulin injection, which was adjusted in the case presented herein according to the patient's feeding schedule, has an important role in controlling the glucose level in infantile DM.
Hypoglycemia is a great concern for the parents of children with diabetes, and especially for the parents of infants and toddlers, who have difficulty communicating illness symptoms and whose clinical signs are non-specific (e.g., poor feeding, lethargy, or jitteriness). The correlation between severe hypoglycemia and the developing brain of infants and toddlers with regard to cognitive impairment remains a controversial area37). Recently, the American Diabetes Association suggested that the acceptable HbA1c level is 7.5%–8.5%, which is recommended for children under 6 years of age3). Hence, the HbA1c level of 7.5%–8.5% was targeted in the case presented herein, and it is believed that the patient was treated properly because his last HbA1c reading was 8.3%.
In conclusion, infants have some age-related features that make the management of their diabetes different from that of older children. Thus, it is necessary to understand the characteristics of new-onset DM in infants or toddlers379). Small numbers of infants and toddlers who have DKA associated with new-onset DM, however, have been reported compared to older children, and this limits the opportunity of individual diabetes clinic teams to experience the management of their conditions. Therefore, collecting data on newly diagnosed diabetes with DKA in infants and toddlers, and realizing active communication between regional and district general hospitals for taking care of this particular age group, should be encouraged.
Notes
Conflict of interest: No potential conflict of interest relevant to this article was reported.