Turner syndrome with spinal hemorrhage due to vascular malformation
Article information
Abstract
Turner syndrome (TS) is a relatively common chromosomal disorder and is associated with a range of comorbidities involving the cardiovascular system. Vascular abnormalities, in particular, are a common finding in cases of TS. However, dissection involving the vertebral arteries is rare. Here, we report the case of a 9-year-old girl with TS who had been treated with growth hormone replacement therapy for the past 3 years. She presented with weakness of both lower legs, and was ultimately diagnosed with spinal hemorrhage due to vascular malformation. We treated her with intravenous high dose dexamethasone (0.6 mg/kg) and she could walk without assistance after 6 days of treatment. In conclusion, when a patient with TS shows sudden weakness of the lower limbs, we should consider the possibility of spinal vessel rupture and try to take spine magnetic resonance imaging as soon as possible. We suggest a direction how to make a proper diagnosis and management of sudden vertebral artery hemorrhage in patients with TS.
Introduction
Turner syndrome (TS) is a relatively common chromosomal disorder affecting approximately 1 in 2,500 live female births1). It is associated with a range of comorbidities involving the cardiovascular system which affects both pediatric and adult patients. Vascular abnormalities, including aortic dilatation, aortic dissection, aortic coarctation, aneurysm, hemangioma, and arteriovenous malformations, are a common finding in cases of TS234). In fact, incidence of cardiovascular anomalies in TS has been reported to be up to 45%5). Aortic dissection, which can occur with or without coarctation, is a rare but potentially life-threatening complication of TS6), however, dissection involving the vertebral arteries is rare. Here, we report the case of a 9-year-old girl with TS who had been treated with growth hormone (GH) replacement therapy and developed spinal hemorrhage due to spinal vascular malformation.
Case report
A 9-year-old girl who was previously diagnosed with TS was referred to our department due to weakness of both lower legs for the past 3 days. She had lymphedema at birth and was diagnosed with 45X/47XXX mosaic TS in the neonatal period. At the time of diagnosis, she received echocardiography and abdominal ultrasonography for evaluation of common complications in TS, and the results were all normal. She demonstrated several characteristics associated with TS, including high-arched palate, low hairline, low-set ears and shield chest, and she had been treated with GH replacement therapy for the past 3 years for management of her short stature. She did not have any family history of cardiovascular disease. Her father's height was 176 cm, her mother's height was 164 cm, and the midparental height was 163.5 cm. Her height was 133 cm (between the 10th and 25th percentile) and body weight was 31.7 kg (between the 25th and 50th percentile). During the 3 years of GH replacement therapy, her height increased by as much as 16.5 cm, with a growth rate of 5.5 cm/yr.
On presentation, her blood pressure was 97/49 mmHg and heart rate was 100 bpm with a regular heartbeat. Cardiac and chest auscultation were normal, and peripheral pulses were symmetric and strong. She did not have a fever or any other signs of infection or inflammation.
Her mental status was alert and there was no evidence of increased intracranial pressure. Motor function decreased to grade 3 in the left lower leg, and to grades 4-5 in right lower leg. She showed decreased dorsiflexion of the left great toe, but plantar flexion was intact. Lower leg sensation and deep tendon reflexes of both knees also were intact.
Results of routine blood examination and coagulation tests were all within normal range. To rule out infection or inflammation, several viral studies of blood and cerebrospinal fluid were performed, and the results were all negative. Serum levels of insulin-like growth factor-1 and insulin-like growth factor binding protein-3 were 487.04 ng/dL and 72,000 ng/dL respectively, which were both within normal range according to her age.
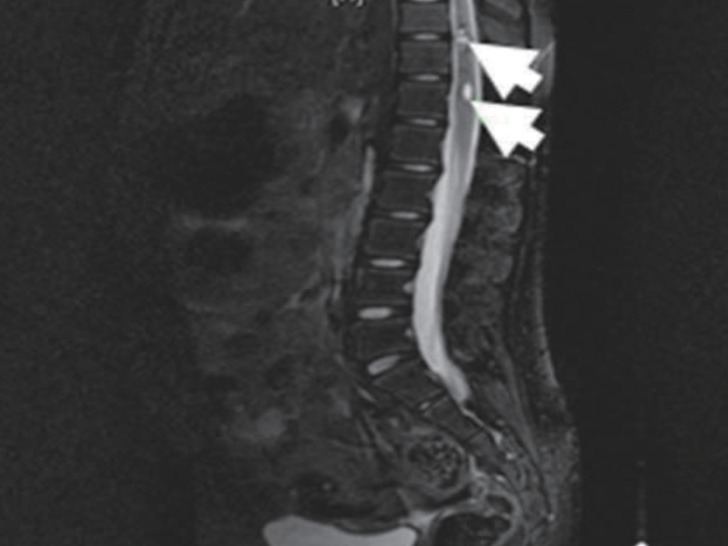
Echocardiography showed no abnormalities. Brain computed tomography performed in the emergency department also showed no abnormalities. Therefore, we performed whole-spine magnetic resonance imaging (MRI) and spinal angiography (Figs. 1, 2). On the whole-spine MRI, T2-weighted images showed acute intradural extramedullary spinal hemorrhage and suspicious vascular malformation at T 11-12. On the spinal angiography, there was no remarkable evidence of spinal artery aneurysm or tumor staining.
T2-weighted magnetic resonance image taken on hospital day 2, shows acute intradural extramedullary spinal hemorrhage (white arrow) at T 11-12.
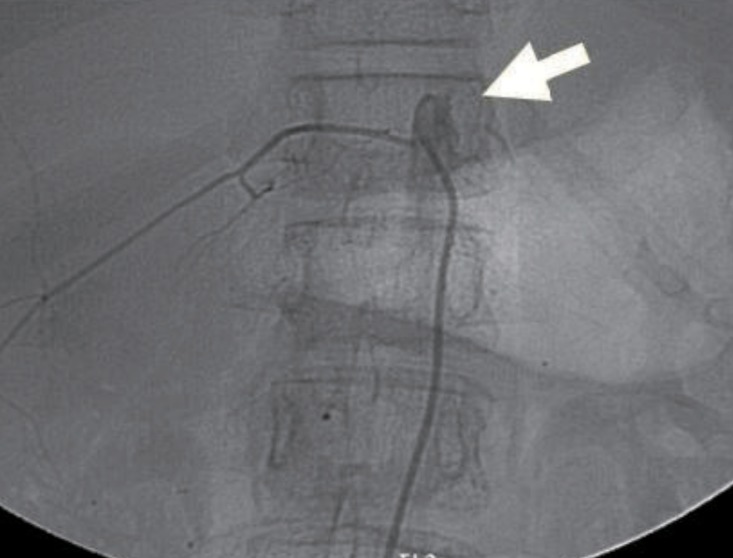
Spinal angiography taken on hospital day 2, shows no remarkable evidence of spinal artery aneurysm or tumor staining (white arrow).
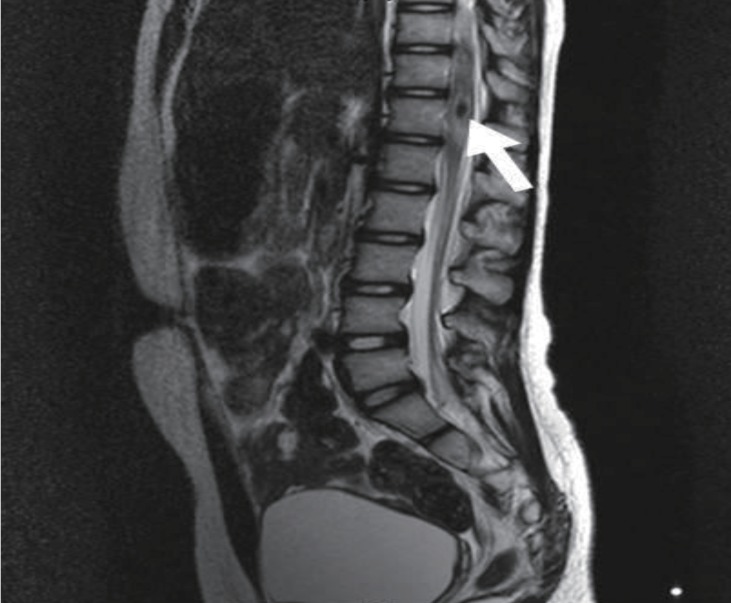
She was treated with intravenous high dose dexamethasone (0.6 mg/kg) for 6 days, and gradually recovered motor function of both lower legs. On hospital day 6, she underwent rehabilitation for standing and walking. On hospital day 9, she could walk without assistance. We tapered the dexamethasone dose over 3 days, and then we changed to oral prednisolone (0.3 mg/kg). On hospital day 13, her motor function was grade 5 in the right lower leg and grades 4-5 in left lower leg. At that point, she was discharged. Follow-up spine MRI performed 7 days later, showed resolving process of the subacute intradural extramedullary hemorrhage and the still existing cavernous malformation at T 11-12 (Fig. 3). After the discharge, she had no problems in walking or standing, and she has been regularly followed up in our outpatient clinic every 6 months. There have been no more emerging symptoms up until her last visit.
Discussion
TS in a phenotypic female patient is characterized by short stature, premature ovarian failure, and congenital cardiovascular defects. Vascular abnormalities, including arteriovenous malformation, are a common finding in cases of TS234). However, spinal hemorrhage has not been reported to our knowledge.
Typical symptoms related to vertebral hemorrhage include neck pain, radicular pain, and myelopathy, such as quadriplegia or quadriparesis7), which occur due to spinal cord compression by the hematoma. Although it can be a neurosurgical emergency, some cases of spontaneous remission after conservative treatment have been reported7). Given the high incidence of aortic dissection in TS, the possibility of cervical vessel dissection should be considered in patients with TS who report sudden weakness of the lower limbs6).
Patients with TS should be treated with recombinant human GH replacement therapy to increase their final height8910). It has been proved that earlier initiation of GH replacement therapy leads to better outcome in final height10). In our case, the patient had been receiving GH replacement therapy for 3 years, so there were some concerns about the potential adverse effects of such therapy on cardiovascular development. However, we could not find a similar report or review of cardiovascular problems in a patient with TS. Cardiovascular problems, such as aortic dissection or rupture, have been reported in patients with TS who are receiving GH replacement therapy, but the relationship or causality remains unlikely9).
Spine MRI and angiography are crucial tools for diagnosis of vertebral hemorrhage and vertebral artery anomaly. MRI can detect intramural hemorrhage and demonstrate the residual patent lumen of the vessel. Emergent imaging techniques using spine MRI and angiography should be considered for proper detection of extracranial artery abnormalities in patients with TS69).
In this report, we described the first case of spinal hemorrhage in a patient with TS that was associated with suspicious vascular malformation. In conclusion, when a patient with TS shows sudden weakness of the lower limbs, we should consider the possibility of spinal vessel rupture and try to take spine MRI as soon as possible. As above, we suggest a direction how to make a proper diagnosis and manage the sudden vertebral artery hemorrhage in patients with TS.
Notes
Conflict of interest: No potential conflict of interest relevant to this article was reported.