Clinical usefulness of the measurement of serum fructosamine in childhood diabetes mellitus
Article information
Abstract
Purpose
Glycosylated hemoglobin (HbA1c) is often used as an indicator of glucose control. It usually reflects the average glucose levels over two to three months, and is correlated with the development of long-term diabetic complications. However, it can vary in cases of hemoglobinopathy or an altered red blood cell lifespan. The serum fructosamine levels reflect the mean glucose levels over two to three weeks. This study was designed to determine the clinical usefulness of the combined measurement of serum fructosamine and HbA1c in the management of childhood diabetes mellitus and the correlation between them.
Methods
Clinical data on 74 Korean children and adolescents with diabetes mellitus who were under management at the Department of Pediatrics of Dankook University Hospital were evaluated. Their fructosamine and HbA1c levels were reviewed based on clinical information, and analyzed using IBM SPSS Statistics ver. 21.
Results
Their HbA1c levels showed a strong correlation with their fructosamine levels (r=0.868, P<0.001). The fructosamine level was useful for the prompt evaluation of the recent therapeutic efficacy after the change in therapeutic modality. It was also profitable in determining the initial therapeutics and for the estimation of the onset of the disease, such as fulminant diabetes.
Conclusion
The measurement of both fructosamine and HbA1c was useful in managing childhood diabetes mellitus, especially when there was discrepancy between the clinical information and the HbA1c level.
Introduction
Uncontrolled diabetes mellitus in childhood and adolescence can result in impairment of physical and emotional development as well as long-term complications including nephropathy, neuropathy and retinopathy1). Therefore, intensive glycemic control is important in managing childhood diabetes mellitus.
HbA1c is often used as an indicator of glucose control over the recent two to three months and is correlated with the development of long-term diabetic complications. It is a widely used method for assessing long term diabetic control2). However, it can vary in cases of hemoglobinopathy or an altered red blood cell (RBC) lifespan. It also does not reflect recent changes in blood glucose in relation to disease management3,4). In contrast, measurement of the concentration of serum glycosylated proteins can be used as a more rapidly responding parameter5). Serum fructosamine, another nonenzymatic glycosylated substance in the blood, reflects the mean glucose levels over the recent two to three weeks. It can be obtained very quickly and is inexpensive to perform. Baker et al.6) reported that estimation of fructosamine concentrations may provide a simple means of screening for diabetes mellitus. Various other studies support the clinical significance of measuring fructosamine in diabetes mellitus as a marker of glycemic variability or glucose control in patients with hemoglobinopathy or renal failure7,8,9). This study was designed to determine the clinical usefulness of measuring serum fructosamine in the management of childhood diabetes mellitus and the correlation between HbA1c and fructosamine levels.
Materials and methods
1. Subjects
A total of 74 Korean diabetic children and adolescents who had undergone laboratory tests (HbA1c, fructosamine, and random glucose) were enrolled in this study. They were under management at the Department of Pediatrics of Dankook University Hospital from December 2009 to December 2012. The subjects were classified into type 1 diabetes mellitus (T1DM) or type 2 diabetes mellitus (T2DM) according to their clinical and biochemical characteristics. The subjects with low serum/urine C-peptide, absolute insulin requirement (>0.5 U/kg/day) for survival, or initial presence of anti-insulin/anti-islet autoantibodies were classified into T1DM, and the others were classified into T2DM. Subjects who had significant anemia or hypoalbuminemia were excluded.
2. Methods
Clinical data were collected retrospectively from a review of the medical records of the study subjects. The clinical and biochemical parameters on their age and gender, the duration of their disease, and their hemoglobin, albumin, HbA1c, and fructosamine levels were reviewed. Their serum albumin, glucose, aspartate aminotransferase (AST), alanine aminotransferase (ALT), blood urea nitrogen (BUN), and creatinine levels as well as fructosamine levels were measured using an modular EVO automated chemistry analyzer (Roche, Basle, Switzerland).
The subjects' HbA1c levels were measured with Bio-Rad Variant II TURBO (Bio-Rad Laboratories, Tokyo, Japan) via high-performance liquid chromatography (HPLC). Their hemoglobin levels were determined through the cyanide-free sodium lauryl sulphate (SLS) method using the Sysmex XE-5000 automated hematology system (Sysmex Corp., Kobe, Japan). Their total T3, free T4, and TSH concentrations were analyzed in a Cobas 6000 (Roche, Tokyo, Japan) autoanalyzer according to the results of an electrochemiluminescence immunological test (ECLIA).
The levels of serum fructosamine and plasma HbA1c, as well as of blood glucose, were reviewed based on clinical information. The correlation among plasma HbA1c, serum fructosamine, and estimated fructosamine levels was investigated, and they were compared with the average glucose level estimated via HbA1c and that estimated via fructosamine.
3. Statistics
All the data were expressed as mean±standard deviaton values. All the statistical analyses were performed using IBM SPSS Statistics ver. 21 (IBM Co., Armonk, NY, USA). Independent samples t-test was used to compare the clinical and biochemical parameters between the T1DM and T2DM groups. To evaluate the relationship between HbA1c and fructosamine, a linear regression analysis was used. A P value of <0.05 was considered statistically significant.
4. Ethics statement
The study was approved by the Institutional Review Board of Dankook University Hospital (IRB No. 2014-07-015). Informed consent was waived by the IRB.
Results
Among 74 patients, 37 were male and 37 were female. A total of 245 samples were assayed for HbA1c and fructosamine. Their mean±standard deviation values were 9.05%±2.56% and 393.53±143.49 µmol/L, respectively. The mean age at the time of diagnosis and assay, and the duration of the disease at assay, were 9.87, 12.94, and 3.31 years, respectively (Table 1). The HbA1c levels showed strong correlations with the fructosamine levels (r=0.868, P<0.001), and were also applied to both T1DM and T2DM groups as well as to both the male and female patients (Fig. 1, Table 2). An equation was formulated to estimate the average plasma glucose levels using the fructosamine levels to evaluate glycemic control (Table 2, Fig. 1). To derive the relationship between HbA1c and the estimated average glucose, the following formula was used: eAG (mg/dL)=(28.7×HbA1c)-6.7, r2=0.8410).
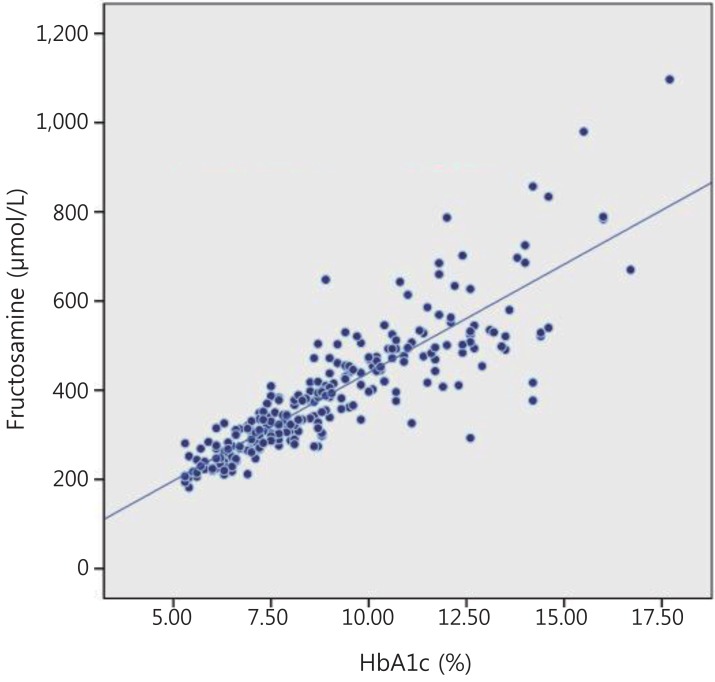
Correlation between serum fructosamine and plasma glycosylated hemoglobin (HbA1c) in children and adolescents with diabetes mellitus. y=48.55x-45.84. r2=0.753.
The correlated formula about estimated fructosamine values was obtained from our results: estimated fructosamine=48.55×HbA1c-5.84 (Fig. 1). This equation, eAG (mg/dL)=0.59×furoctosamine (µmol/L)-9.6, is considered useful in evaluating recent clinical findings and the efficacy of the treatment (Table 3). Because the fructosamine levels indicated the average glucose concentration over the previous two to three weeks, the measurement of serum fructosamine was useful in selecting the initial therapeutics and promptly evaluating the therapeutic efficacy after a change in therapeutic modality (Table 3). It was also useful in estimating the onset of the disease, e.g., fulminant diabetes (Table 3).
These are four typical cases that show the clinical usefulness of fructosamine in the management of diabetes mellitus. In case 1, there was no hyperglycemic symptom such as polyuria, polydipsia, nocturia, thirst, or weight loss before the onset of nonketotic hyperosmolar coma. On the first visit to Emergency Department, the plasma glucose level was 723 mg/dL. The level of HbA1c was 5.6%, and the fructosamine level was 244 µmol/L (normal range, 205-85 µmol/L). The hemoglobin was 4.4 g/dL and the fasting serum C-peptide was 0.8 ng/mL. The patient was managed with insulin based on a diagnosis of fulminant diabetes and was discharged with improved condition. In case 2, the patient visited Emergency Department with symptoms such as vomiting, headache, and general weakness. Her laboratory test revealed 499 mg/dL of serum glucose, positive serum ketone, and 7.26 of blood pH. Her HbA1c and serum fructosamine were 7.5% and 409 µmol/L. A day before the onset of her symptoms, she was out camping and omitted insulin injection in the evening. From the biochemical and clinical features, we could assume that her average glucose levels during the recent three to four months, and the recent two to three weeks were 168 mg/dL and 221.7 mg/dL, respectively, although her serum glucose level upon visit was 499 mg/dL. We considered these findings were indicative of the recent worsening of her glycemic control and the aggravation to diabetic ketoacidosis due to omission of insulin injection. In case 3, the obese (body mass index 29.5 kg/m2, >97 percentile) patient was diagnosed as T2DM in a regular health screening, one month prior to his admission. He had been controlling his diabetes through balanced diet and physical exercise until his admission. On admission, his plasma HbA1c, serum fructosamine, and fasting serum glucose levels were 11.9%, 408 µmol/L, and 247 mg/dL, respectively. His estimated average glucose levels over the recent three to four months and for the recent two to three weeks were 297.7 mg/dL and 221.1 mg/dL, respectively. We concluded that he did not yet require insulin treatment. Therefore, metformin medication was started. Three months later, his plasma HbA1c became 7.1% with metformin medication. In the fourth case, the patient had been experiencing weight loss, polyuria, and polydipsia for 8 months. Four days prior to admission, he had fever, diarrhea, and lower extremity weakness. His initial blood glucose was 303 mg/dL and urinalysis revealed positive urine glucose. His HbA1c, fructosamine, serum insulin, and C-peptide were 11.5%, 586 µmol/L, 10.5 µIU/mL, and 2.1 ng/mL, respectively. It can be assumed that his diabetes mellitus was aggravated by his recent infection. Therefore, we added insulin therapy for management.
Discussion
It is well known that intensive control of blood glucose in diabetes mellitus is very important in the prevention of acute complications such as diabetic ketoacidosis as well as chronic complications such as nephropathy, retinopathy, and neuropathy. For better control of diabetes mellitus, it is important to maintain balanced energy intake, active exercise, regular insulin injection or medication of oral hypoglycemic agents as well as frequent blood glucose monitoring. A minimum of four daily blood glucose measurements should be performed, although more frequent blood glucose monitoring may be needed in some children and adolescents. Because of the difficulties in balancing insulin injections with the activities and diet, as many as 31% of all children with T1DM were reported to experience one or more episodes of severe hypoglycemia11,12). This happens more commonly in children with good controlled diabetes mellitus11,13).
Glycemic biomarkers are used as important tools in determining whether metabolic control has been kept within the target range. They are also surrogate markers for estimating the risk of chronic complications such as nephropathy, retinopathy, and neuropathy. HbA1c is considered the gold standard for the measurement of glycemic control. HbA1c levels reflect the mean glucose levels over the recent two to three months. HbA1c is highly positively correlated with the occurrence of chronic diabetic complications, and interventions that reduce HbA1c correspondingly reduce the risk of complications14,15,16,17). Both observational studies18,19) and controlled clinical trials15,17,18) have demonstrated the strong correlation between HbA1c and retinopathy, as well as between HbA1c and other microvascular complications of diabetes mellitus. However, use of HbA1c poses some limitations. Conditions that affect the RBC lifespan will affect the HbA1c results. RBCs that have a short lifespan secondary to destruction (i.e., hemolytic anemia, destruction via passage through abnormal heart valves, or splenomegaly) will reduce the level of HbA1c which is independent of the mean serum glucose levels20,21,22). Hemoglobinopathies such as sickle cell traits, and other abnormal hemoglobin variants such as hemoglobin C and E, can lead to falsely high or low HbA1c values, depending on the laboratory methodology used20,23,24). In the negative iron balance status, iron and hemoglobin deficiencies can be followed by deficient RBC production which translates to a slow turnover of RBCs. In this situation, more time for glycosylation of RBCs falsely increases the HbA1c values. Patients with chronic kidney disease present anemia of multifactorial etiology including erythropoietin deficiency, decreased RBC survival, decreased response of marrow precursor cells to erythropoiesis signals, and iron deficiency20,25,26). The uremic state also affects the accuracy of the HbA1c assay. In uremia, there are direct interactions with glycosylated hemoglobin analyses and uremic conditions induce hemoglobin modification to carbamylated hemoglobin which interferes with the laboratory analysis. Moreover, HbA1c has a limited ability to reflect short-term glycemic changes, and cannot separately reflect postprandial hyperglycemia and fasting hyperglycemia9,27). More evidences suggest that postprandial hyperglycemia and glycemic variability may be independent risk factors of macrovascular complications in patients with diabetes mellitus9,27,28,29).
For better control of diabetes mellitus to prevent acute or chronic complications, frequent surveillance is beneficial, especially in children. Therefore, another biomarker indicating short-term glucose control will be required. Fructosamine, glycosylated albumin, and 1,5-anhydroglucitol (1,5-AG) have been drawing attention for use in populations whose HbA1c levels may be difficult to interpret30,31,32,33), such as those with anemia, hemolysis, or renal disease30,34,35,36). Fructosamines are circulating biomarkers that reflect short-term glucose control in DM37). Fructosamine is a measure of glycosylated serum proteins, the most common of which is albumin. Fructosamine levels are correlated with the average glucose levels in the previous 10-4 days, and can be used clinically as complementary markers of short-term changes in glucose management37). Baker et al.38) reported that concentrations of fructosamine appeared more useful in monitoring short-term changes after alterations in the management of diabetes. In addition, measurement of fructosamine offers many advantages of technical simplicity, low cost, and ease of automation using standard laboratory equipment6).
Several studies have evaluated the association between fructosamine and postprandial hyperglycemia or glycemic fluctuations6,9,38). When the treatment modality is changed, fructosamine is useful because it reflects short-term glucose control6,9,38,39). The four cases presented in the paper (Table 3) illustrate the usefulness of measuring fructosamine levels in the management of childhood diabetes mellitus. The fructosamine level is useful in the prompt evaluation of the recent therapeutic efficacy after a change in therapeutic modality. It is also useful in determining the initial treatment for children and adolescents with diabetes mellitus and in estimating the onset of disease, such as fulminant diabetes. Fulminant type 1 diabetes mellitus can be defined as diabetes mellitus in which the process of β-cell destruction is extremely fast, resulting in fulminant progression of hyperglycemia to ketoacidosis. Hanafusa and Imagawa40) reported the screening and diagnostic criteria for fulminant type 1 diabetes. In case 1, the patient was managed based on a diagnosis of fulminant diabetes mellitus defined by normal ranges of HbA1c and fructosamine levels. Her fasting serum C-peptide level was 0.8 ng/mL on admission, but it became 0.1 ng/mL, 17 months later. The plasma HbA1c and serum fructosamine levels were 7.4% and 341 µmol/L, respectively, 8 months after the diagnosis. The patient was still on insulin therapy, therefore it can be concluded that the patient had fulminant diabetes mellitus at the time of diagnosis.
In conclusion, the combined measurement of both serum fructosamine and plasma HbA1c is useful in the management of childhood diabetes mellitus, especially when there is discrepancy between the clinical information and the HbA1c level.
Notes
Conflict of interest: No potential conflict of interest relevant to this article was reported.


