 |
 |
- Search
| Ann Pediatr Endocrinol Metab > Volume 19(3); 2014 > Article |
Abstract
Purpose
Methods
Results
Acknowledgments
References
Fig.┬Ā1

Fig.┬Ā2
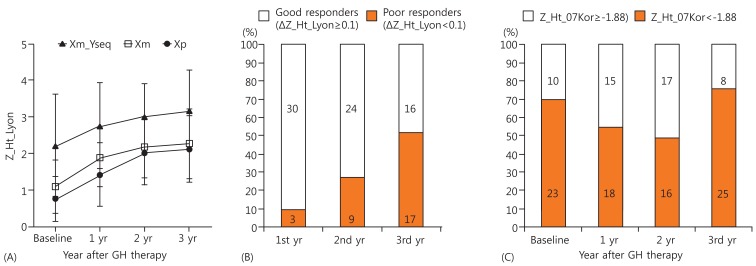
Fig.┬Ā3
Table┬Ā1.
| Variable | Xp (n=10) | Xm (n=15) | Xm_Yseq (n=8) | P-value |
|---|---|---|---|---|
| CA at start GH (yr) | 7.2┬▒3.0 | 9.0┬▒3.4 | 9.2┬▒2.6 | 0.344 |
| BA at start GH (yr) | 5.8┬▒2.8 | 8.3┬▒3.4 | 8.3┬▒2.9 | 0.149 |
| BA delay (yr) | 1.39┬▒1.12 | 0.73 ┬▒1.58 | 0.84┬▒0.60 | 0.427 |
| Z_MPH | ŌĆō0.36┬▒0.90 | ŌĆō0.72┬▒1.01 | ŌĆō0.13┬▒0.51 | 0.245 |
| Z_Ht_Lyon | 0.75┬▒0.61a) | 1.08┬▒0.74a) | 2.21┬▒1.39a) | 0.047 |
| Z_Ht_07Kor | ŌĆō2.5┬▒0.61 | ŌĆō2.44┬▒0.71 | ŌĆō1.75┬▒1.03 | 0.193 |
| Z_BMI_07Kor | ŌĆō0.17┬▒1.57 | 0.78┬▒0.88 | 0.81┬▒0.96 | 0.070 |
Values are presented as mean┬▒standard deviation.
The differences in the means of continuous variables (baseline Z_Ht_Lyon) with heterogenous variance between the 3 groups were tested by WelchŌĆÖs analysis of variance (ANOVA).
TS, Turner syndrome; CA, chronological age; GH, growth hormone; BA, bone age; BA delay, difference between CA and BA; Z_MPH, midparental height z-score; Z_Ht_Lyon, height z-score according to Turner syndrome specific height reference by Lyon et al. [12]; Z_Ht_07Kor, height z-score according to the 2007 Korean reference height values; Z_BMI, body mass index z-score.
Table┬Ā2.
Values are presented as mean┬▒standard deviation.
The differences in the means of nonnormally distributed variables (GH dose IU/kg/wk (0ŌĆō3 yr), GH dose IU/kg/wk (0ŌĆō1 yr), GH dose IU/kg/wk (1ŌĆō2 yr), and GH dose IU/kg/wk (2ŌĆō3 yr) were tested by Kruskal-Wallis testb). Other variables between the 3 groups were normally distributed. Analysis of variance was used for other variables with homogeneous variance. The difference in the means of variables within 2 subsets was analyzed using the Bonferroni method with P set at 0.025 (a)P<0.025).
GH, growth hormone; Z_Ht_Lyon, height z-score according to Turner syndrome specific height reference by Lyon et al. [12]; Z_Ht_07Kor, height z-score according to the 2007 Korean reference height values; Z_BMI, body mass index z-score; ΔZ_Ht_Lyon, change in Z_Ht_Lyon; and HV, height velocity.
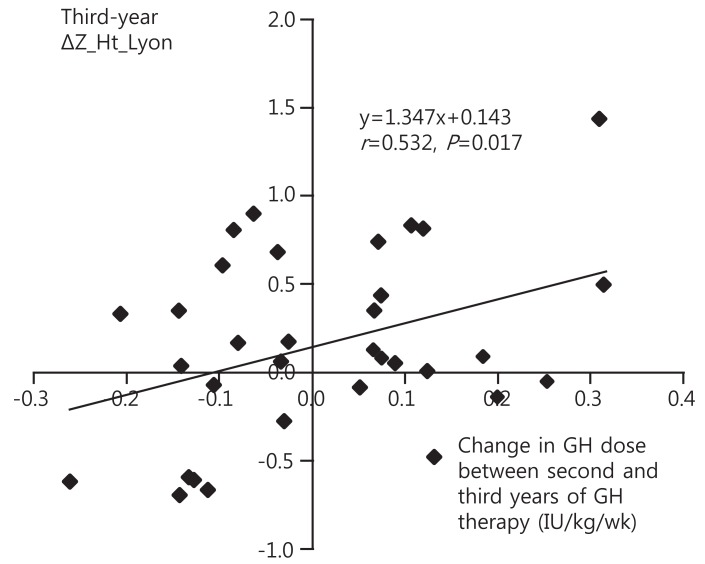
Table┬Ā3.
S.E, Standard error; GH, growth hormone; Z_Ht_Lyon, height z-score according to Turner syndrome specific height reference by Lyon et al. [12]; BA, bone age; BA delay, difference between CA and BA; Xp, paternally derived X chromosome; Xm, maternally derived X chromosome; Xm_Yseq, 45,X/46,X,+mar(Y); Z_MPH, midparental height z-score; First- and second-year ΔZ_Ht_Lyon, first- and second-year change in Z_Ht_Lyon (first- and secondyear height response).
- TOOLS
-
METRICS

-
- 4 Crossref
- 9,062 View
- 102 Download
- Related articles in APEM





