The difference in serum alkaline phosphatase levels between girls with precocious puberty and those with normal puberty
Article information
Abstract
Purpose
Serum alkaline phosphatase (ALP) level is the most valid marker of bone formation. Precocious puberty (PP) in girls is characterized by early growth acceleration. The aim of this study was to determine whether serum ALP levels differ between girls with PP and those with normal puberty, and whether ALP level varies with age or Tanner stage.
Methods
This retrospective study included girls with PP (n=61) and normal puberty (n=71) who visited the outpatient clinic at Department of Pediatrics, Chungnam National University Hospital from March 2010 to August 2011. We obtained age, height, parental height, weight, bone age, Tanner stage, and concentrations of luteinizing hormone, follicular-stimulating hormone, estradiol, ALP, and insulin-like growth factor-1 (IGF-1) from the participants' medical records.
Results
Age and predicted adult height were significantly lower in girls with PP than in those with normal puberty. The height standard deviation score (SDS), weight SDS, body mass index, midparental height, bone age, and IGF-1 level were higher in girls with PP than in those with normal puberty. ALP level was significantly higher in 5- to 8-year-old girls with PP than in age-matched girls with normal puberty. The mean ALP levels were higher in girls with PP than bone age-matched girls with normal puberty (P=0.0003)
Conclusion
Serum ALP level showed the significance differences between girls with PP and those with normal puberty. The reasons for and the mechanisms underlying this elevation in serum ALP level in girls with PP should be investigated further.
Introduction
One of the hallmarks of puberty is growth in stature. A key element of puberty is a second growth spurt that is characterized by markedly increased bone turnover and rate of bone growth. A previous study showed that bone turnover and bone growth are maximal in midpuberty (Tanner stage 3) and decrease toward the adult level in late puberty (Tanner stage 4-5)1). A substantial pubertal growth spurt can occur in girls with precocious puberty (PP), which is defined as early breast development emerging before 8 years of age2). PP is associated with extreme sensitivity of the growth plate to the actions of estrogens; the auxological consequences of PP often precede the signs of sexual maturation, and PP can cause premature fusion of the growth plates3).
Serum alkaline phosphatase (ALP) level is recognized as an indicator of osteoblastic activity and is used widely in routine biochemical tests because it can be measured in almost all laboratories4,5).
The aim of the present study was to investigate the interrelationships between pubertal development and biochemical markers of bone formation (serum ALP level) to evaluate whether bone-formation activity differs between girls with PP and those with normal puberty and whether the differences are associated with age and Tanner stage.
Materials and methods
1. Subjects
The study protocol was performed after informed consent was obtained from the patients and their legitimate guardians. One hundred thirty-two girls (61 PP patients and 71 non-PP controls) were selected from among the children who visited the outpatient - short stature and PP clinic in the Department of Pediatrics, Chungnam National University Hospital, between March 2010 and August 2011.
The subjects were classified into PP and non-PP groups. PP was defined as either early menarche before the age of 10 years or Tanner stage breast 2 or greater for breast development before 8 years of age accompanied by bone age (BA) advanced by at least 1 year compared with chronological age (CA) and a serum luteinizing hormone (LH) level >5.0 IU/L in the course of a gonadotropin-releasing hormone (GnRH) stimulation test. Girls aged ≥9 years who had no breast development before 9 years of age and no advanced BA (≤1 year less than CA), or girls <9 year-old with Tanner stage breast 1 and no advanced BA, or who had achieved menarche at an age ≥13 years were included as non-PP controls. Girls who had an underlying disorder or history of medication such as steroids, growth hormone, or GnRH analogues, or who were overweight or obese (body mass index [BMI]≥85th percentile) were excluded.
2. Methods
The subjects' medical records were reviewed retrospectively, and information on age, height, body weight, pubertal stage (using Tanner stage for breasts), parental height, the presence of illness, and past illness were recorded. Height was measured using a Harpenden stadiometer accurate to the nearest 0.1 cm during a visit to the clinic. Body weight was recorded to the nearest 0.1 kg with an electric scale. Pubertal stage according to Marshall and Tanner was determined by one pediatric endocrinologist at each visit. Midparental height (MPH) was the mean of the parental heights minus 6.5 cm. BMI was calculated as weight (kg)/height (m)2. The standard deviation scores (SDSs) of height, weight, and BMI were calculated using the 2007 growth reference tables for Korean children and adolescents of the Korean Pediatric Society and Korea Centers for Disease Control and Prevention6). BA was determined using Greulich and Pyle's atlas7).
Blood samples were drawn for measurement of the levels of estradiol (E2), basal LH and follicle-stimulating hormone (FSH), peak LH and FSH after a GnRH stimulation test, insulin-like growth factor-1 (IGF-1), liver function test, electrolytes (calcium, phosphorus), and thyroid function test. Serum LH and FSH levels were measured at baseline and 15, 30, 45, 60, and 90 minutes after intravenous bolus administration of GnRH (100 µg Relefact, Sanofi-Aventis, Frankfurt am Main, Germany). A stimulated LH value>5 IU/L is considered to indicate a pubertal response to the GnRH-stimulation test8-10). The serum levels of LH, FSH, and E2 were measured by electrochemiluminescence immunoassays, and IGF-1 by a chemiluminescence immunoassays. Serum ALP level was measured using using Bayer Reagent Packs on an automated chemistry analyzer (Advia 1650 Autoanalyzer, Bayer Diagnostics, Leverkusen, Germany). We considered the manufacturers' details for all assays.
3. Statistics
The numeric data are expressed mean±standard deviation or median (range). We analyzed the differences in auxological and clinical data between groups using Student t-test. Differences in serum ALP levels between the PP and non-PP groups were analyzed according to age and Tanner stage using the Mann-Whitney U test. The mean serum ALP levels between the PP and BA-matched non-PP groups were analyzed using two way analysis of variance with Graphpad prism (GraphPad Software Inc., San Diego, CA, USA). Data analyses were performed using IBM SPSS ver. 20.0 (IBM Co., Armonk, NY, USA), and a P-value <0.05 was considered significant.
Results
1. Auxological and laboratory manifestations
The baseline clinical and laboratory characteristics of the study participants are shown in Table 1. A total of 132 girls (61 girls with PP and 71 with normal puberty) were enrolled in the trial. The age (P<0.001) and predicted adult height (P<0.001) were significantly lower in the PP group than in the non-PP group. The height SDS (P<0.001), weight SDS (P<0.001), BMI (P=0.046), MPH (P=0.009), BA (P=0.006), and IGF-1 level (P=0.005) were higher in the PP group than in the non-PP group. However, height, weight, BMI-SDS, and basal LH, FSH, estradiol, and aspartate aminotransferase, alanine aminotransferase, calcium, and phosphorous levels did not differ significantly between the PP and non-PP groups.
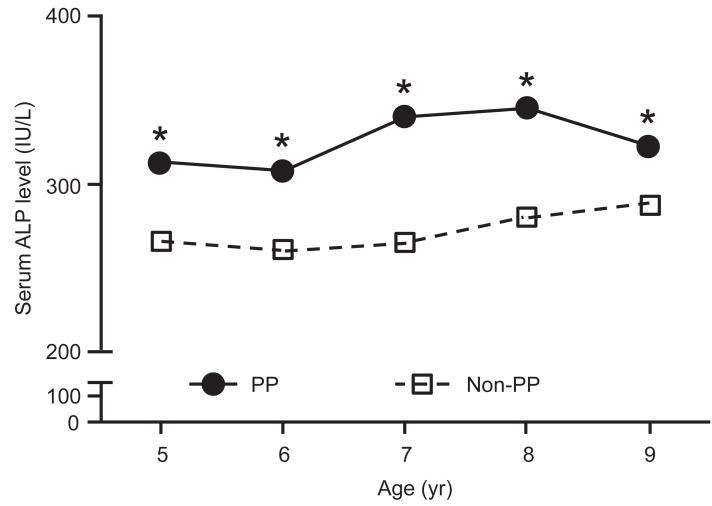
2. Difference in serum ALP levels between the PP and non-PP groups according to age
The age-related changes in serum ALP levels in the PP and non-PP groups are shown in Table 2 and Fig. 1. Two 8-year-old and five 9-year-old girls in the PP group were diagnosed with early menarche and Tanner stage 4. Serum ALP level was significantly higher in 5- to 8-year-old girls in the PP group than in girls of the same age in the non-PP group (P<0.05). At age 9 years, the difference between the two groups was not significant (P=0.065).
3. Difference in serum ALP levels between the PP and non-PP groups according to BA
The BA-related differences in serum ALP levels in the PP and non-PP groups are shown in Fig. 2. The mean serum ALP level was significantly higher in girls with PP group than in girls of the same bone aged non-PP group (P=0.0003).
Discussion
Serum ALP levels were higher in the girls with PP than in the controls. The peak ALP level was observed in Tanner stage 3 in the controls but in Tanner stage 4 in PP patients. We have tried to clarify how changes in serum ALP level according to age and Tanner stage in girls with PP was compared to those with normal puberty, whether serum ALP level was the biomarker to help the additional diagnostic information at initial visit in outpatient clinic.
PP is one of the most common endocrine disorders seen by primary care clinicians and continues to be a major cause of concern for both parents and health care providers. The diagnosis of PP is based on the medical history, physical examination, pubertal staging, and BA. The GnRH-stimulation test is the gold standard for proving premature activation of the hypothalamic-pituitary-gonadal axis11,12). Rapid growth velocity is suggested as the most useful predictor of a positive result on the GnRH-stimulation test, indicating that the clinician can decide the proper timing of the GnRH-stimulation test by estimating the growth velocity13). However, the following visit may be needed to evaluate patient's growth velocity.
PP is manifested by early bone maturation14-16). Biochemical markers of bone metabolism exhibit dynamic changes during both bone growth and remodeling, and their measurement may be useful in the assessment of metabolic bone diseases and growth disorders in children14,17). Bone markers are classified according to the processes they reflect, namely formation and resorption. The levels of bone formation markers increase during different phases of osteoblast development and reflect osteoblast function and bone formation. These markers include ALP, bone-specific ALP, osteocalcin, and procollagen 1 peptides.
Total serum ALP activity is used as a biochemical marker of bone formation to confirm osteoblastic activity in primary hyperparathyroidism, rickets, osteomalacia, and Paget disease. Because ALP is a marker of osteoblastic activity, its level is higher in growing children than in fully grown adults, and the highest ALP level is detected during the rapid growth phases of childhood such as infancy and puberty18). A few study for bone markers in children and adolescents have been demonstrated that serum ALP and bone-specific ALP showed same course with age and Tanner stage, and higher bone specific ALP values at the beginning of puberty Tanner stage 2, aged 10-12 years18,19).
The ALP levels in the non-PP controls in this study correspond well with those of an earlier study showing that, in girls, serum ALP levels are at a maximum in midpuberty Tanner stage 2-3 but decrease in late puberty Tanner stage 3-420). We also found that the girls with PP had higher levels of serum ALP than controls after adjustment for age. The ALP levels in the 9-year-old subgroup did not differ significantly between the PP and non-PP groups, probably because of the small sample size (Table 2). Our findings suggest that, among girls with suspected early breast budding, those with a high serum ALP level are more likely to be in PP compared to same-aged girls.
Interestingly, the mean serum ALP levels significantly differed between the patients and controls by BA-matched study. This cause is not known, but the degree of bone formation between PP and non-PP girls may be some different according to age or BA.
Our study has some limitations. This was a cross-sectional, retrospective study with a small sample size. We did not measure individual longitudinal changes in ALP levels in the girls with PP. The serum ALP level did not adjusted by serum vitamin D level. And we used the total ALP instead of bone-specific ALP.
In conclusion, among girls with suspected early breast budding, those with a high serum ALP level are more likely to be in PP compared to same-aged and same-bone aged girls. Further study is needed to understand the reasons for the difference in serum ALP levels between girls with PP and those with normal puberty according to BA.
Notes
No potential conflict of interest relevant to this article was reported.