Changes of antithroglobulin antibody in children with congenital hypothyroidism
Article information
Abstract
Purpose
It has been reported that antithroglobulin (anti-TG) antibody is increased in the sera of both children with transient congenital hypothyroidism and their mothers. And transplacental transport of thyroid autoantibody was proposed as the pathogenesis of transient congenital hypothyroidism. However this is not known in nontransient congenital hypothyroidism. This study was done to see changes of anti-TG antibody in children with nontransient congenital hypothyroidism.
Methods
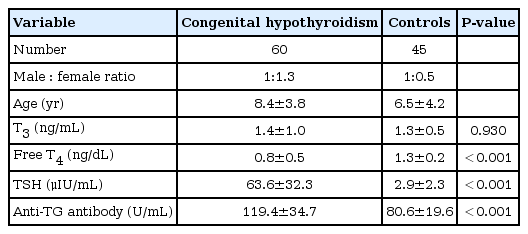
Study patients consisted of 60 patients diagnosed as congenital hypothyroidism in the Department of Pediatrics, Kyungpook National University Children's Hospital, Daegu, Republic of Korea between January 2010 and March 2013. Healthy control were 45 children showing normal thyroid function. Anti-TG antibody and various laboratory tests were analyzed retrospectively, and compared in both children with congenital hypothyroidism and controls.
Results
Anti-TG antibody was significantly higher in children with congenital hypothyroidism compared to healthy controls, 119.4±34.7 U/mL versus 80.6±19.6 U/mL, respectively (P<0.001). There was no significant difference of anti-TG antibody in gender and age.
Conclusion
We observed a significant increase of anti-TG antibody in children with nontransient congenital hypothyroidism compared to healthy controls. Further study focusing pathogenetic role of anti-TG antibody in nontransient congenital hypothyroidism is necessary. Furthermore, the clinical significance in the course of congenital hypothyroidism need to be known.
Introduction
Congenital hypothyroidism is the most common congenital endocrine disorder in childhood, with an incidence of 1:4,000 live births1). Distinctive symptoms are often not manifested in many cases and this leads to delay in diagnosis and treatment. Patients with congenital hypothyroidism often suffer from sequela including delayed bone maturation, growth and development, intelligence disorder, motor disorder, speech impairment, and others2). Late treatment during infancy, in particular, could generate irreversible brain injury and neurological sequela due to delayed development in the central nervous system2,3). A leading form of acquired hypothyroidism is Hashimoto disease and its major clinical symptom is thyroid goiter without tenderness. The pathogenesis of Hashimoto disease is known as abnormal activation of T lymphocyte and the destruction of thyroid tissues caused by antiperoxidase antibody (anti-TPO Ab), thyroid autoimmune antibody, or antithyroglobulin antibody (anti-TG Ab)4).
The authors of this study incidentally experienced an higher level of anti-TG Ab in children with nontransient congenital hypothyroidism during their followed up workups including anti-TG Ab at outpatient clinic. The aim of this study is to compare children with healthy control group to verify an increase of anti-TG Ab in children with nontransient congenital hypothyroidism.
Materials and methods
Study patients consisted of patients diagnosed as congenital hypothyroidism outpatiently in the Department of Pediatrics, Kyungpook National University Children's Hospital from January 2010 to March 2013. Subjects were retrospectively examined with sex, age, T3, free T4, thyroid stimulating hormone (TSH) and anti-TG Ab. The causes of congenital hypothyroidism were classified with thyroid ultrasonography and thyroid scan. Control group comprised 45 patients with normal thyroid function without auto-immune disorders by taking age and sex of patient group with congenital hypothyroidism into consideration. Children diagnosed at the age less than 2 years old was excluded from study patients to differentiate transient congenital hypothyroidism and nontransient congenital hypothyroidism. Hypothyroidism was defined by low levels of T4 or free T4 by age, and an elevated TSH level5). Analysis was performed using Brahms TSH 1 radioimmunoassay (RIA) kit (Thermo Fisher Scientific, Bremen, Germany) for TSH, RIA-gnost T3 kit (Cisbio Bioassays, Codolet, France) for T3, fT4 RIA kit (Beckman Coulter, Brea, CA, USA) for free T4, and Thyroglobulin IRMA kit (Cisbio Bioassay, Codolet, France) for anti-TG Ab. Statistical analysis was conducted using PASW ver. 18.0.0 (IBM Co., Armonk, NY, USA) and P-values of less than 0.05 were considered statistically significant.
Results
A total of 60 children with congenital hypothyroidism comprised 26 boys and 34 girls with the ratio of boys to girls of 1:1.3 and mean age was 8.4±3.8 years. Control group involved a total of 45 patients comprising 30 boys and 15 girls with the ratio of boys to girls of 1:0.5. Mean age was 3.1±3.3 years (Table 1). Among those patients, the causes of congenital hypothyroidism were identified in 44 out of 60 subjects. The causes were thyroid dyshormonogenesis in 26 patients (59%), thyroid dysgenesis in 13 patients (30%), thyroid agenesis in 4 patients (9%), and ectopic thyroid in a 1 patient (2%). The result of thyroid function test in the initial diagnosis of nontransient hypothyroidism children is shown in Table 1. Their serum T3 level (ng/mL) was 1.4±1.0, showing no statistically significant difference compared to 1.3±0.5 of control group (P=0.930). Serum free T4 level (ng/dL) was 0.8±0.5 in congenital hypothyroidism group, and was significantly lower compared to 1.3±0.2 of control group (P<0.001). TSH level (µIU/mL) was 63.6±32.3 in congenital hypothyroidism group, and was statistically significant higher compared to 2.9±2.3 of control group (P<0.001) (Table 1). Among 60 patients with congenital hypothyroidism, anti-TG Ab level (U/mL) was 119.4±34.7, and was significant higher compared to 80.6±19.6 of control group (P<0.001) (Table 1).
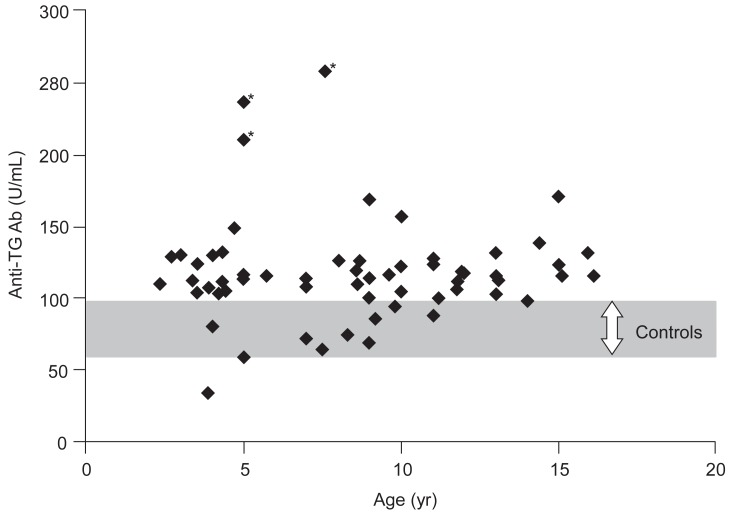
Anti-TG Ab level (U/mL) was examined by sex in chlidren with congenital hypothyroidism, anti-TG Ab levels were 114.5±31.4 in boys and 121.3±35.8 in girls, showing no statistically significant difference. There was no statistically significant difference in patient group with congenital hypothyroidism by age (Fig. 1).
Discussion
Thyroid hormone plays crucial role in development and differentiation of the central nervous system, physical growth and pubertal development, dental and skeletal growth, metabolism, and functional maintenance of organs6). Congenital hypothyroidism is classified as the most common endocrine disorder in childhood showing the deficiency of thyroid hormone from the birth with an incidence of 1/4,000 newborns according to the screening test of Fisher1). The major causes of congenital hypothyroidism are thyroid dysgenesis or biosynthetic defects of thyroid hormone7,8). Congenital hypothyroidism is divided into nontransient or transient according to duration of illness. Thyroid function is known to recover several months to years after birth in transient hypothyroidism8). Distinctive symptoms are often not presented in many cases and this leads to delay in diagnosis and treatment9,10). Late treatment during infancy, in particular, could incur irreversible brain injury and neurological sequela due to delayed development of central nervous system2,3). The newborn screening for congenital hypothyroidism was first performed in Quebec city, Canada and Pittsburg, the United States in 1972, and the diagnosis rate of congenital hypothyroidism has almost doubled11). Republic of Korea has conducted the newborn screening for congenital hypothyroidism from the early 1990's12).
Leading acquired thyroid disorders are Graves disease and Hashimoto disease. Significant auto-antibodies are anti-TPO Ab, anti-TG Ab, anti-TSH receptor antibody (anti-TSHR Ab), and others in acquired thyroid disorders. Among those, anti-TPO Ab and anti-TG Ab take very crucial role in diagnosing Graves disease and Hashimoto disease13). Moreover, those autoantibodies may increase in autoimmune diseases including systemic lupus erythematosus or rheumatoid arthritis, in addition to thyroid disorders14). Thyroglobulin is a 670 kDa protein composed of 2,768 amino acids, and known as a clinically useful indicator in autoimmune thyroid disorders and thyroid cancer. And anti-TG Ab could be showed in about 11% of normal individuals15). The authors of this study was able to identify a significant increase of anti-TG Ab in patient group with nontransient congenital hypothyroidism which is not acquired thyroid disorders compared to healthy control group. In the past, the transplacental movement of maternal anti-thyroid auto-antibodies had been suggested as the cause of congenital hypothyroidism as hypothesis. Bona et al.16) performed screening test of anti-TG Ab, antimicrosomal antibody, and TSH blocking antibody (TSH-blocking Ab) in 18 newborns with congenital hypothyroidism and their 14 mothers. Among those, both antimicrosomal Ab and anti-TG Ab increased in 5% of all newborns and their mothers. In addition, TSH-blocking Ab increased in a mother and her newborn. Authors were interpreted the result of this study by the movement of IgG from mothers to newborns through placenta, and it influenced the thyroid development of infant. Toublanc et al.17) performed screening test for thyroid auto-antibodies in 42 newborns with congenital hypothyroidism and their mothers. Among those, TSH binding inhibitor antibody increased in 38% of newborns. However, there was no association with the results of their mothers. Antibody-dependant cell mediated cytotoxicity and thyroid growth immunoglobulin block accounted for 24% of all patients, respectively. The association with maternal results reportedly accounted for 90% in the former one and 84% in the latter one. Ordookhani et al.18) tested for thyroid hormone, TSHR Ab, anti-TPO Ab, and anti-TG Ab in mothers and their newborns with transient hypothyroidism. Newborns with hypothyroidism were treated thyroid hormone orally and showed normal thyroid function three years after gradual suspension of medication administration. Among 35,067 newborns conducted with screening test, 6 newborns were confirmed with transient hypothyroidism, and anti-TG Ab was positive in 4 out of 6 subjects. In control group, anti-TG Ab was positive only in 6 out of 106 subjects. Anti-TG Ab level was significantly higher in patient group than control group. Anti-TPO Ab was found to be negative in all subjects in transient hypothyroidism group. Sutherland et al.19) and Goldsmith et al.20) reported that pregnant women with autoimmune hypothyroidism could generate hypothyroidism in newborns due to the transplacental movement of auto-antibodies.
This study observed that anti-TG Ab significantly increased in patient group with nontransient congenital hypothyroidism unlike transient hypothyroidism compared to control group. Significant increases were shown in anti-TG Ab levels in all disorders of thyroid dysgenesis, thyroid dyshormonogenesis and thyroid agenesis regardless of different causes of congenital hypothyroidism. We think our study is a pilot one showing increased anti-TG Ab titer in nontransient congenital hypothyroidism, because we could not find any literature on the change of anti-TG Ab in children with nontransient congenital hypothyroidism. The hypothesis of higher anti-TG Ab titer in children with nontransient congenital hypothyroidism is that antigenicity related to thyroid tissue or proteins would be associated with the pathogenesis of nontransient congenital hypothyroidism. Three study patients showing the highest titers of anti-TG Ab has agenetic thyroid (Fig. 1). It can be said that higher titers of anti-TG Ab in children with thyroid agenesis may support our hypothesis in part.
There are some limitations in the study. Our study showed the most common cause of congenital hypothyroidism was dyshormonogenesis rather than dysplasia of thyroid gland. We thought this is because of small number of study patients and/or limitation of one institute survey. And we did not analyze changes of anti-TG Ab in series. Another limitation was that the level of thyroid auto-antibodies was not assessed. In order to differentiate transient congenital hypothyroidism and nontransient congenital hypothyroidism, we excluded patients under 2 years of age from study patients. Anti-TG Ab was significantly higher in patients with nontransient congenital hypothyroidism instead of transient hypothyroidism. Therefore, additional studies are thought to be necessary to survey the relationship between thyroid autoimmune antibodies such as anti-TG Ab and the incidence of nontransient congenital hypothyroidism. Furthermore, more studies are thought to be crucial to investigate anti-TG Ab and clinical progression.
In conclusion, anti-TG Ab significantly increased in chlidren with nontransient congenital hypothyroidism. Further studies are necessary to investigate the effect of thyroid autoimmune antibodies on the incidence and clinical progress of congenital hypothyroidism.
Notes
No potential conflict of interest relevant to this article was reported.