 |
 |
- Search
| Ann Pediatr Endocrinol Metab > Volume 22(1); 2017 > Article |
|
Abstract
Acknowledgments
References
Fig. 1
Structures of major perfluoralkyl substances. PFBA, perfluorobutanoic acid; PFPeA, perfluoropentanoic acid; PFHxS, perfluorohexane sulfonic acid; PFHpA, perfluoroheptanoic acid; PFOA, perfluorooctanoic acid; PFNA, perfluorononanoic acid; PFDA, perfluorodecanoic acid; PFDoDA, perfluorododecanoic acid; PFTrDA, perfluorotridecanoic acid; PFTeDA, perfluorotetradecanoic acid; PFBS, perfluorobutane sulfonic acid; PFOS, perfluorooctane sulfonic acid; PFDS, perfluorodecane sulfonic acid.
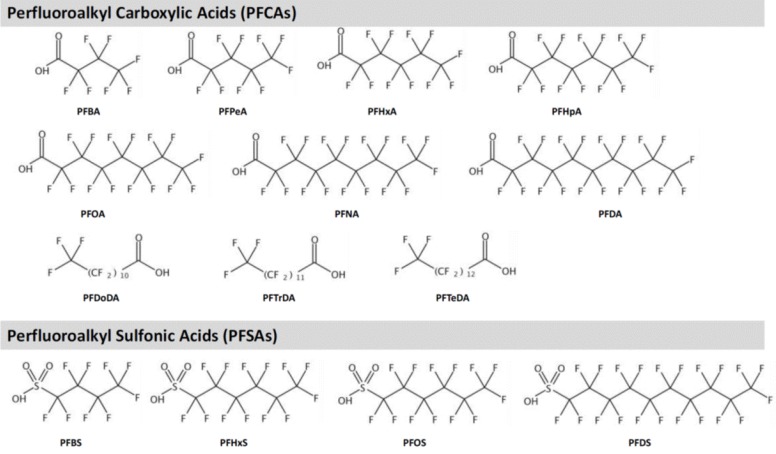
Table 1
Perfluoroalkyl substances - classification and physicochemical characteristics
CAS RN, chemical abstracts service registry number.
*Serum elimination half-lives in human. †Serum elimination half-lives in rat. a)Buck et al. (2011)11). b)At 25℃, calculated using Advanced Chemistry Development (ACD/Labs) Software V11.02 (© 1994–2016 ACD/Labs). c)Chang et al. (2008)13). d)Nilsson et al. (2010)14). e)Olsen et al. (2007)15). f)Olsen et al. (2009)16). g)Chengelis et al. (2009)17). h)Ohmori et al. (2003)18). i)Tatum-Gibbs et al. (2011)19). j)Sundström et al. (2012)20). k)Chang et al. (2012)21).
Table 2
Associations between exposure to PFASs and thyroid hormones among nationally representative populations of United States
| PFASs | Population age (n) | Study period | Thyroid measure | Association | Reference |
|---|---|---|---|---|---|
| PFOA PFOS | General population (3,974) | 1999–2006 | Current thyroid disease |
-PFOA highest quartile shows odd ratio (OR) for current thyroid disease of 2.24 (95% CI, 1.38–3.65) in females, and 2.12 (0.93–4.82) for male -PFOS highest quartile shows OR for current thyroid disease of 2.68 (1.03–6.98) in males |
Melzer et al. (2010)25) |
| PFOS, PFOA, PFDeA, PFHxS, PFNA | >12 years (1,832) | 2007–2008 | fT3, fT4, tT3, tT4, TSH |
-PFOA in positive associations with TSH and tT3 -PFHxS in positive association with tT4 |
Jain (2013)29) |
| PFOA, PFHxS, PFNA, PFOS | >18 years (1,525) | 2007–2008 | fT3, fT4, tT3, tT4, TSH |
-Among population with normal range of TPOAb and urinary iodine (n=1,012), PFOA in positive association with fT3 -Among population with abnormal range of TPOAb and urinary iodine (n=26), PFHxS and PFOA in negative associations with fT4. All 4 PFSAs in positive associations with fT3, tT3, fT3/fT4, or TSH |
Webster et al. (2016)28) |
| PFOA, PFHxS, PFNA, PFOS | ≥20 years (1,181) | 2007–2010 | fT3, fT4, tT3, tT4, TSH |
-Among female (n=509), PFOA in positive association with tT3; PFHxS in positive associations with tT4 and tT3 -Among male (n=672), PFHxS in negative association with fT4 |
Wen et al. (2013)26) |
| PFOA, PFHxS, PFNA, PFOS | ≥12 years (1,682) | 2011–2012 | fT3, fT4, tT3, tT4, TSH |
-No associations observed in whole population -Among females of 20–40 years of age (n=268), all PFSAs in positive associations with TSH. But in females of 60-80 years of age, PFHxS in negative association with TSH -Among female juveniles (n=145), PFOA in negative association with TSH |
Lewis et al. (2015)27) |
Table 3
Associations between exposure to PFASs and thyroid hormones among pregnant women or infants
| PFASs | Study population | Study period | Thyroid measure | Association | Reference |
|---|---|---|---|---|---|
| PFHxS, PFOA, PFOS, PFNA, PFDeA, PFUnDA, PFDoDA, PFHpA, PFHxA | Taiwan Maternal and Infant Cohort Study (285 mothers at 3rd trimester, and 116 cord blood) | 2000–2001 | fT4, tT4, tT3, TSH |
-Among mothers, PFHxS in positive association with TSH; maternal PFNA, PFUnDA, or PFDoDA in negative associations with maternal fT4 and tT4 -Maternal PFNA, PFUnDA, or PFDoDA in negative associations with cord tT4 and tT3; Maternal PFDeA in negative association with cord tT3. |
Wang et al. (2014)38) |
| PFOS, PFOA | Hokkaido Study on the Environment and Children's Health (392 pairs) | 2002–2005 | fT4, TSH |
-PFOS in negative association with maternal TSH, but positive association with cord TSH -PFOA shows no association (mother during 24 weeks of gestation and 5 days after delivery) |
Kato et al. (2016)22) |
| PFOS, PFOA, PFHxS, PFNA, PFUnDA, PFHpS, PFDeA | Northern Norway Mother and Child Cohort Study. Pregnant women (930) | 2003-2004 | TSH | -PFOS in positive association with TSH (18 weeks of gestation) | Wang et al. (2013)37) |
| PFOS, PFOxS, PFOA |
Pregnant women, 2nd trimester (96 cases, 175 controls) Edmonton, Alberta, Canada |
2005–2006 | Hypo-thyroidism | -Noassociation | Chan et al. (2011)43) |
| PFOA, PFHxS, PFNA, PFOS | Euthyroid pregnant women (152) Vancouver, Canada | 2007–2008 | fT4, tT4, TSH |
-PFNA in positive association with TSH -Among TPOAb normal group, no association -Among TPOAb abnormal group (n=14), PFNA, PFOS, or PFOA in positive associations with TSH; all PFSAs in negative associations with fT4 |
Webster et al. (2014)35) |
| 26 PFSAs | Northern Norway Mother and Child Contaminant Cohort Study. 2nd trimester (441) | 2007–2009 | fT3, fT4, tT3, tT4, TSH, TBG, TTR, Albumin, TBI, TPOAb |
-PFOS in positive association with TSH -PFDA or PFUnDA in negative association with T3 |
Berg et al. (2015)36) |
| PFHxS, PFHpS, PFOS, PFOA, PFNA, PFDeA, PFUnDA, PFTrDA | Pregnant women (n=44) and infant (n=43) pair | 2008-2009 | tT3, tT4, TSH |
-Maternal PFOS in negative association with cord tT3 -Maternal PFOA in positive association with cord TSH -Maternal PFTrDA in negative associations with cord tT4 and tT3 |
Kim et al. (2011)42) |
| PFOA, PFOS, PFTrDA, PFHxS, PFUnDA, PFNA, PFPeA, PFDeA, PFDoDA, PFTeDA | Korea Ewha Birth & Growth Retrospective Cohort (279) | 2006–2010 | T3, T4, TSH |
-Among female infants, PFPeA, or PFHxS in positive association with T3 or T4; PFNA in negative association with TSH -Among all infants, PFPeA in positive association with T4 (PFSAs measured in maternal serum of 24–28 weeks of gestations. Thyroid hormones measured in cord blood serum) |
Shah-Kulkarni et al. (2016)41) |
| PFOA, PFOS, other POPs | Netherlands LINK study (83 pairs) | 2011–2013 | T4 in heel prick blood spots |
-Among girls (n=31), PFOA in positive association with T4 -Among boys, no associations |
de Cock et al. (2014)40) |
| PFHxS, PFOS, PFOA, PFNA, PFDeA, PFUnA, PFDoA |
Beijing, China, Mother-infant pair (157) |
2013 | fT3, fT4, tT3, tT4, TSH |
-Maternal PFNA, PFDeA, PFUnDA, PFDoDA, or PFOS in negative associations with maternal TSH; Maternal PFDoA in negative associations with fT3, tT3, fT4, or tT4 -Cord PFOA, PFNA, PFDeA, PFUnDA, PFDoDA, PFHxS, or PFOS in negative associations with maternal tT3; cord PFOS in negative association with maternal TSH |
Yang et al. (2016)39) |
PFAS, perfluoroalkyl substance; PFHxS, perfluorohexane sulfonic acid; PFOA, perfluorooctanoic acid; PFOS, perfluorooctane sulfonic acid; PFNA, perfluorononanoic acid; PFDeA, perfluorodecanoic acid; PFUnDA, perfluoroundecanoic acid; PFDoDA, perfluorododecanoic acid; PFHpA, perfluoroheptanoic acid; PFHxA, perfluorohexanoic acid; PFTrDA, perfluorotridecanoic acid; PFPeA, perfluoropentanoic acid; PFHpS, perfluoroheptane sulfonate; PFTeDA, perfluorotetradecanoic acid; PFUnA, perfluoroundecanoic acid; PFDoA, perfluorododecanoic acid; TSH, thyroid stimulating hormone; fT3, free triiodothyronine; fT4, free thyroxine; TPOAb, thyroid peroxidase antibody .
- TOOLS
- Related articles in APEM







