Turner syndrome with primary hyperparathyroidism
Article information
Abstract
Turner syndrome has multiple comorbidities such as osteoporosis, obesity, diabetes, hypothyroidism, and hypertension. As they are treatable conditions in Turner syndrome, early recognition and proper treatment should be needed. We report on a 23-year-old woman with Turner syndrome who presented with severe osteoporosis and hypercalcemia. Laboratory tests showed elevated levels of serum calcium and parathyroid hormone. Dual-energy X-ray absorptiometry showed severe osteopo-rosis (z score, -3.5). Ultrasound and 99mTc scintigraphy of parathyroid glands showed an adenoma in the right inferior gland. She was diagnosed with primary hyperparathyroidism due to an adenoma of the parathyroid gland. After excision of the adenoma, the patient's serum calcium and parathyroid hormone levels returned to normal. Although only a few cases of Turners syndrome with primary hyperparathyroidism have been reported, hyperparathyroidism should be considered in cases of Turner syndrome with severe osteoporosis and hypercalcemia.
Introduction
Turner syndrome is the most common chromosomal abnormality in females. It occurs in 1/2,500 to 1/3,000 live-born females and results from a total or partial absence of the X chromosome1). The clinical manifestations are diverse with common features like short stature and amenorrhea. Turner syndrome is commonly diagnosed because of short stature in midchildhood, primary amenorrhea in adolescence, or in some cases, typical cardiac anomalies during infancy. Turner syndrome is accompanied by multiple medical problems such as cardiovascular diseases, kidney diseases, autoimmune thyroid diseases, diabetes mellitus, osteoporosis, and inflammatory bowel disease. Hearing loss and psychosocial problems are also common in Turner syndrome2,3).
Hyperparathyroidism is characterized by the overactivity of the parathyroid glands. It can be classified as primary or secondary hyperparathyroidism. Primary hyperparathyroidism is a disease caused by an increase in parathyroid hormone secretion without a specific stimulus leading to hypercalcemia. The most common cause is single gland adenoma in postmenopausal women. Secondary hyperparathyroidism is caused by low blood calcium levels resulting in elevation of parathyroid hormone, for instance, vitamin D deficiency, chronic renal disease, and malabsorption, etc.4).
The authors diagnosed primary hyperparathyroidism caused by parathyroid adenoma in 23-year-old patient with Turner syndrome based on incidental identification of hypercalcemia and severe osteoporosis.
Case report
A 23-year-old woman with Turner syndrome was hospitalized because of incidentally identified hypercalcemia during routine blood tests at out-patient clinic. She was diagnosed with Turner syndrome (45,X) due to short stature at age 11 years and had received recombinant human growth hormone (rhGH) for about 4 years. Since she was 13 years, she has been on estrogen priming and estrogen-progesterone cyclic therapy.
Her vital signs were normal with blood pressure of 112/74 mmHg, heart rate of 80 bpm, respiratory rate of 20/min, and body temperature of 36℃. She was short in stature despite long-term therapy and overweight, measuring 151.2 cm (standard deviation score [SDS], -2.093) and weighing 55.4 kg (SDS, -0.367). She showed typical signs of Turner syndrome, such as high arched palate, webbed neck, shield chest deformity, and cubitus valgus on both sides. Mild scoliosis was noted in simple T-L spine radiographs. Tanner stage was breast III and pubic hair II.
The laboratory profiles demonstrated normal complete blood count and electrolytes. Serum calcium and ionized calcium levels were elevated to 11.2 mg/dL (8.4 to 10.2 mg/dL) and 5.07 mg/dL (3.9 to 4.5 mg/dL), respectively, while phosphorous and alkaline phosphatase were normal (2.6 mg/dL and 107 IU/L, respectively). Thyroid stimulating hormone and free T4 were both within normal range (3.3 µU/mL and 1.4 ng/dL, respectively). Serum parathyroid hormone level increased up to 159 pg/mL (9 to 65 pg/mL), whereas 25-hydroxyvitamin D3 decreased to 15.1 ng/mL (20 to 100 ng/mL). Hypergonadotropic hypogonadism was present in spite of estrogen/progesterone replacement therapy (follicle stimulating hormone of 32.4 mIU/mL, luteinizing hormone of 10.5 mIU/mL, and estradiol of 21.2 pg/mL). Urinalysis indicated mild occult hematuria. Increase in both calcium/creatinine (Cr) ratio (0.211 mg/mg; Cr, <0.14 mg/mg) and phosphorus/Cr ratio (0.985 mg/mg; Cr, 0.21 to 0.75 mg/mg) were observed.
Additional tests were carried out to exclude multiple endocrine neoplasia, however, all of the tests were normal: serum and urine catecholamine level and calcitonin were within normal range and abdomen ultrasonography did not show any specific findings in the pancreas and adrenal glands, nor any stone-related abnormalities in both kidneys. Dual-energy X-ray absorptiometry (DEXA) scan revealed osteoporosis with bone mineral density (BMD) of the spine (L1-L4) (0.736 g/cm2; z score, -3.5) and femur (0.651 g/cm2; z score, -2.7). In parathyroid scintigraphy, a locally increased activity in the lower right lobe of a parathyroid was not washed out even after 2 hours (Fig. 1). An oval-shaped, low attenuated, hypervascular mass in the lower pole of the right parathyroid was observed in neck ultrasonography measuring 1.2 cm×0.8 cm×0.6 cm, suggesting parathyroid adenoma.
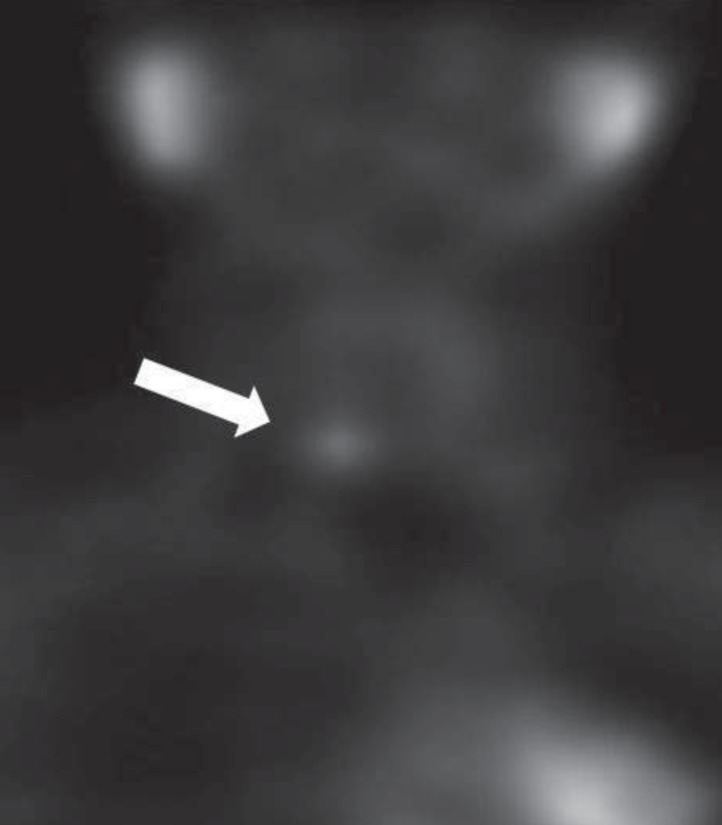
Parathyroid scintigraphy of the patient demonstrated adenoma in the right inferior parathyroid gland (white arrow).
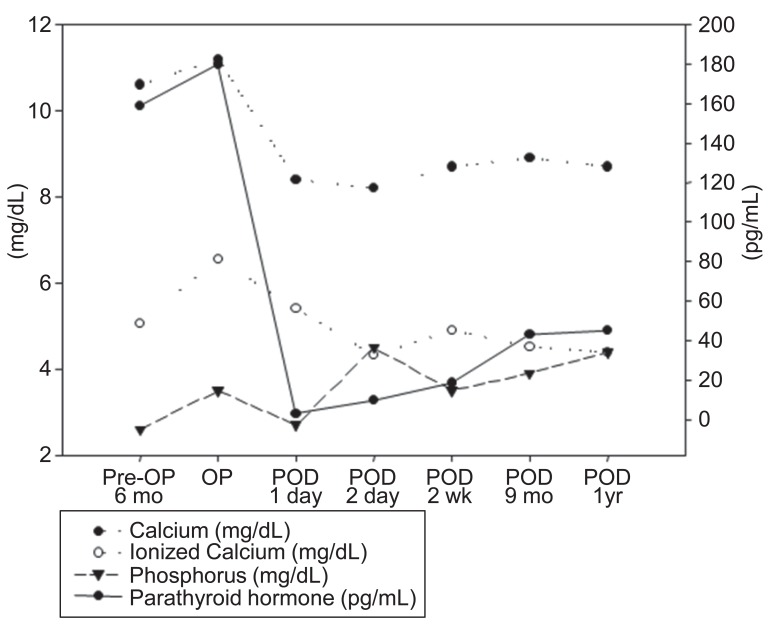
The lower right parathyroid gland was completely resected, and pathologic findings confirmed the diagnosis of parathyroid gland adenoma measuring 1.3 cm×0.9 cm×0.8 cm (Fig. 2). Two days after operation, serum calcium level, ionized calcium and parathyroid hormone levels were normalized (Fig. 3). After discharge, the subject has been monitored by laboratory tests such as serum chemistry and urine calcium/Cr ratio until now and follow up image is under considered.
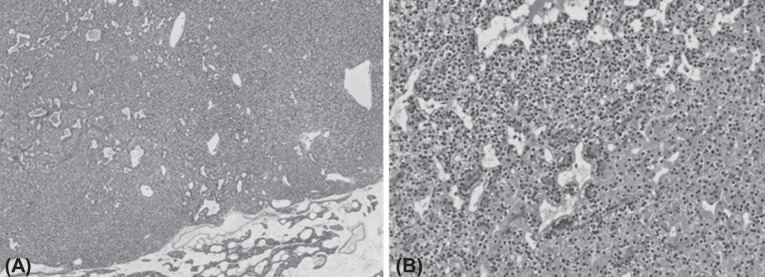
Microscopic findings of the excised parathyroid adenoma of patient. (A) The tumor is well circumscribed and lack of stromal fat, which is abundant in the normal gland at the periphery. The tumor cells are arranged in solid sheets or follicular structure (H&E, ×40). (B) The tumor is predominantly composed of chief cells intermingled with oxyphilic cells (H&E, ×200).
Discussion
This study reports primary hyperparathyroidism in an adult patient with Turner syndrome, which has been reported to be extremely rare5-9). Life-long monitoring is required in patients with Turner syndrome because of its various clinical manifestations such as cardiovascular disease, autoimmune thyroid disease, diabetes, and deafness2). Osteoporosis in Turner syndrome is caused by estrogen deficiency resulting from gonadal dysgenesis, genetic factors related to osteogenesis in the X chromosome such as SHOX gene haploinsufficiency, and decrease in androgen and vitamin D insufficiency10-13). In previous studies, it has been reported that osteoporosis occurs more frequently in patients with Turner syndrome compared to normal control13,14). In patients with Turner syndrome under estrogen replacement therapy, volumetric BMD was similar to that of normal control15). However, without proper and timely estrogen treatment, bone density drops due to rapid loss of trabecular bone tissue and incidence of fractures increased16). Therefore, it is important to conduct regular monitoring of BMD and estrogen replacement therapy to prevent osteoporosis in patients with Turner syndrome17). The previous study in Denmark demonstrated that DEXA measurements revealed that 28% of adult Turner syndrome patients showed osteopenia and 23% of osteoporosis13). In addition, serum calcium and 25-hydroxyvitamin D3 levels decreased and parathyroid hormone (PTH) level increased compared to control13). Decrease in 25-hydroxyvitamin D3 levels in Turner syndrome may be due to a lower degree of physical activity resulting in the lack of sunlight exposure and insufficient uptake of vitamin D18).
This case showed persistent hypercalcemia as well as severe osteoporosis, which is very unusual in Turner syndrome. It was turned out that she had primary hyperparathyroidism due to parathyroid gland adenoma. Primary hyperparathyroidism contributed to aggravating her osteoporosis. To date, only a few Turner syndrome patients have been reported to be associated with primary hyperparathyroidism. Including our case, only 6 cases of primary hyperparathyroidism in Turner syndrome have been reported5-9). Most patients manifested osteoporosis, hyperacalcemia, and parathyroid adenoma (Table 1). The underlying mechanism of development remained to be discovered. Low 25-hydroxyvitamin D levels are related to higher plasma PTH levels and subsequently exacerbate primary hyperparathyroidism19,20).
In conclusion, we noticed primary hyperparathyroidism in Turner syndrome patients presented with hypercalcemia and severe osteoporosis. It should be considered that the patients with Turner syndrome with persistent hypercalcemia and severe osteoporosis might have primary hyperparathyroidism.
Notes
No potential conflict of interest relevant to this article was reported.