 |
 |
- Search
| Ann Pediatr Endocrinol Metab > Volume 27(4); 2022 > Article |
|
Abstract
Purpose
Methods
Results
Notes
Funding
This study was partially supported by a grant from the Korea Institute of Radiological and Medical Sciences (KIRAMS), funded by the Ministry of Science and ICT (MSIT), Republic of Korea. (No.50541-2019).
Fig. 1.

Fig. 2.
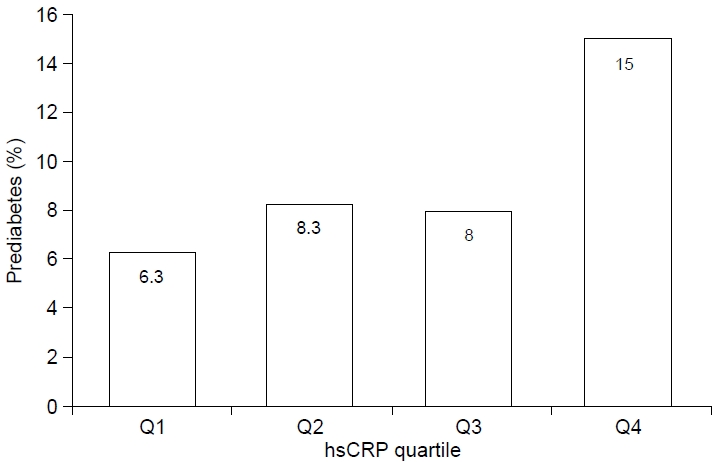
Table 1.
Values are presented as mean±standard deviation.
MetS, metabolic syndrome; hsCRP, high-sensitivity C-reactive protein; BMI, body mass index; WC, waist circumference; SBP, systolic blood pressure; DBP, diastolic blood pressure; FPG, fasting plasma glucose; TG, triglyceride; HDL-C, high-density lipoprotein cholesterol; HbA1c, glycosylated hemoglobin; TC, total cholesterol; LDL-C, low-density lipoprotein cholesterol.
Metabolic syndrome is defined as at least 3 of the following 5 criteria: WC ≥90th percentile, SBP or DBP ≥90th percentile, FPG ≥100 mg/dL, TG ≥110 mg/dL, and HDL-C ≤40 mg/dL.
Table 2.
| Variable |
Quartiles of hsCRP (mg/dL)† |
P-value | |||
|---|---|---|---|---|---|
| Q1 (<0.27) | Q2 (0.27–0.37) | Q3 (0.37–0.70) | Q4 (≥0.70) | ||
| Number | 284 | 330 | 317 | 316 | |
| Metabolic syndrome (%) | 2.1 | 2.1 | 4.4 | 15.5 | <0.001 |
| Abdominal obesity (%) | 3.5 | 3.3 | 9.1 | 31.3 | <0.001 |
| High blood pressure (%) | 7.4 | 11.5 | 15.8 | 22.8 | <0.001 |
| Hyperglycemia (%) | 9.5 | 12.4 | 12.6 | 13.6 | 0.461 |
| Hypertriglyceridemia (%) | 19.4 | 15.8 | 20.8 | 28.8 | 0.001 |
| Low HDL-C (%) | 6.0 | 7.6 | 12.3 | 19.0 | <0.001 |
| HbA1c ≥5.7 % (%) | 6.3 | 8.3 | 8.0 | 15.0 | 0.001 |
| LDL ≥130 mg/dL (%) | 9.2 | 5.8 | 8.5 | 9.2 | 0.331 |
| No. of MetS components | 0.46±0.74 | 0.51±0.79 | 0.71±0.88 | 1.16±1.22 | <0.001 |
Table 3.
hsCRP, high-sensitivity C-reactive protein; MetS, metabolic syndrome; HDL-C, high-density lipoprotein cholesterol; HbA1c, glycosylated hemoglobin; LDL-C, low-density lipoprotein cholesterol.
Model 1, adjusted for age and sex; Model 2, adjusted for age, sex, and other components of the metabolic syndrome.
Model 2† in analysis for HbA1c≥5.7, adjusted for age, sex, abdominal obesity, high blood pressure, hypertriglyceridemia, and low HDL-C.
References
- TOOLS
- Related articles in APEM







