 |
 |
- Search
| Ann Pediatr Endocrinol Metab > Volume 26(2); 2021 > Article |
|
Abstract
Purpose
Methods
Results
Notes
ACKNOWLEDGMENTS
Fig.ô 1.
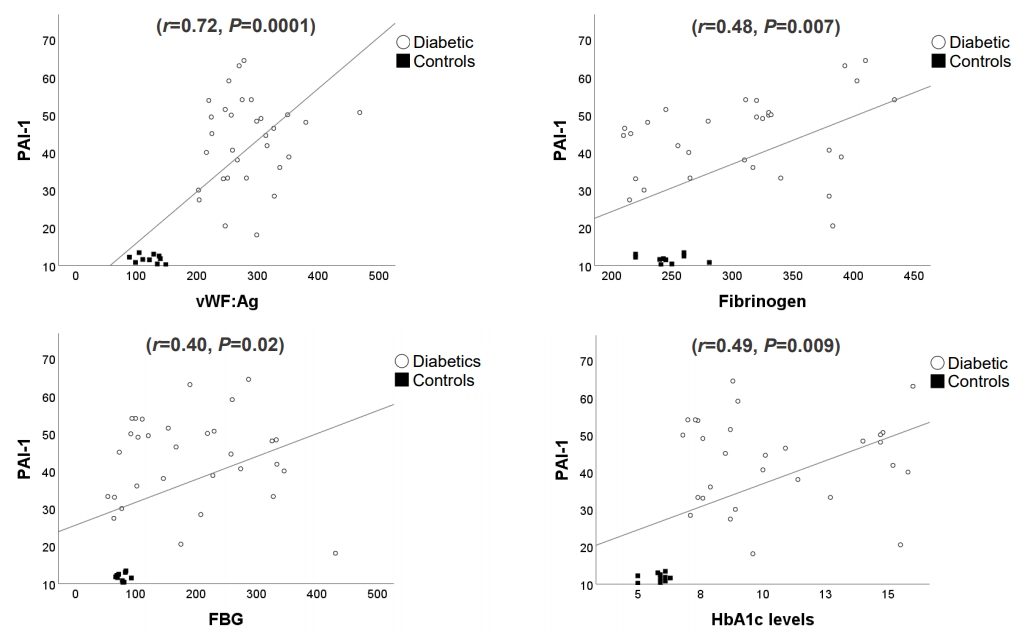
Tableô 1.
| Characteristic | Diabetic (n=35) | Control (n=20) | P-value |
|---|---|---|---|
| Sex, male:female (n) | 20:15 | 13:7 | 0.567 |
| Age (yr), median (IQR) | 11 (10ã13) | 10 (9ã12) | 0.137 |
| BMI (kg/m2) | 19.4ôÝ4.0 | 18.7ôÝ1.5 | 0.630 |
| Diabetes evolution (yr) | 3.7ôÝ2.0 | - | - |
| FBG (mg/dL) | 210ôÝ109 | 77ôÝ8 | 0.0001* |
| HbA1c (%) | 10.4ôÝ3.0 | 5.8ôÝ0.4 | 0.0001* |
| TC (mg/dL) | 167ôÝ39 | 156ôÝ28 | 0.414 |
| HDL-C (mg/dL) | 45ôÝ12 | 42ôÝ8 | 0.471 |
| LDL-C (mg/dL) | 107ôÝ39 | 99ôÝ30 | 0.569 |
| Triglyceride (mg/dL) | 90ôÝ48 | 87ôÝ16 | 0.788 |
| PT (%) | 86ôÝ15 | 90ôÝ9 | 0.468 |
| aPTT (seg) | 45ôÝ6 | 42ôÝ5 | 0.163 |
| Platelet count (û103/ö¥L) | 286ôÝ37 | 209ôÝ50 | 0.539 |
| Fibrinogen (mg/dL) | 308ôÝ66 | 246ôÝ18 | 0.0001* |
| PAI-1 (ng/mL) | 41.6ôÝ12.0 | 11.7ôÝ1.0 | 0.0001* |
| vWF:Ag (%) | 284ôÝ55 | 121ôÝ19 | 0.0001* |
Values are presented as meanôÝstandard deviation unless otherwise indicated.
IQR, interquartile range; BMI, body mass index; FBG, fasting blood glucose; HbA1c, glycosylated hemoglobin A1c; TC, total cholesterol; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; PT, prothrombin time; aPTT, activated partial thromboplastin time; PAI-1, plasminogen activator inhibitor-1; vWF:Ag, Von Willebrand Factor:Antigen.
Tableô 2.
| Variable | GGC (n=9) | PGC (n=26) | P-value |
|---|---|---|---|
| FBG (mg/dL) | 123.13ôÝ62.51 | 253.44ôÝ102.96 | 0.004* |
| HbA1c (%) | 7.2ôÝ0.3 | 11.5ôÝ2.7 | 0.0001* |
| PT (%) | 88ôÝ8 | 84ôÝ18 | 0.636 |
| aPTT (sec) | 45ôÝ3 | 46ôÝ8 | 0.619 |
| Platelet count (û103/ö¥L) | 274ôÝ47 | 290ôÝ36 | 0.492 |
| Fibrinogen (mg/dL) | 341ôÝ64 | 290ôÝ63 | 0.056 |
| PAI-1 (ng/mL) | 43.8ôÝ12.1 | 40.4ôÝ13.3 | 0.545 |
| vWF:Ag (%) | 272ôÝ39 | 288ôÝ58 | 0.630 |
Values are presented as meanôÝstandard deviation.
GGC, good glucemic control (HbA1cãÊ8%); PGC, poor glucemic control (HbA1c>8%). FBG, fasting blood glucose; HbA1c, glycosylated hemoglobin A1c; PT, prothrombin time; aPTT, activated partial thromboplastin time; PAI-1, plasminogen activator inhibitor-1; vWF:Ag: Von Willebrand Factor:Antigen.
Tableô 3.
References
- TOOLS
- Related articles in APEM
-
Islet cell transplantation transitioning to proven therapy for type 1 diabetes2021 June;26(2)
Commentary on "Haemostatic state in children with type 1 diabetes"2021 June;26(2)
Thyroid imaging study in children with suspected thyroid dysgenesis2021 March;26(1)
Cardiac autonomic neuropathy in nonobese young adults with type 1 diabetes2019 September;24(3)







