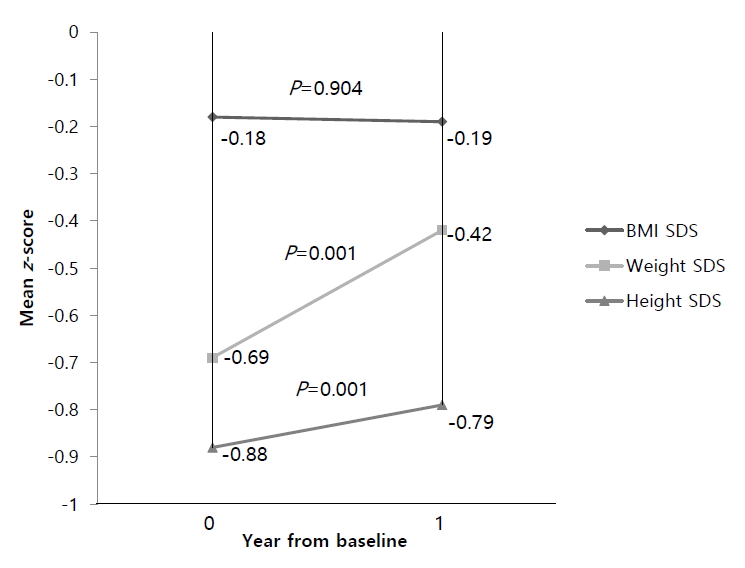
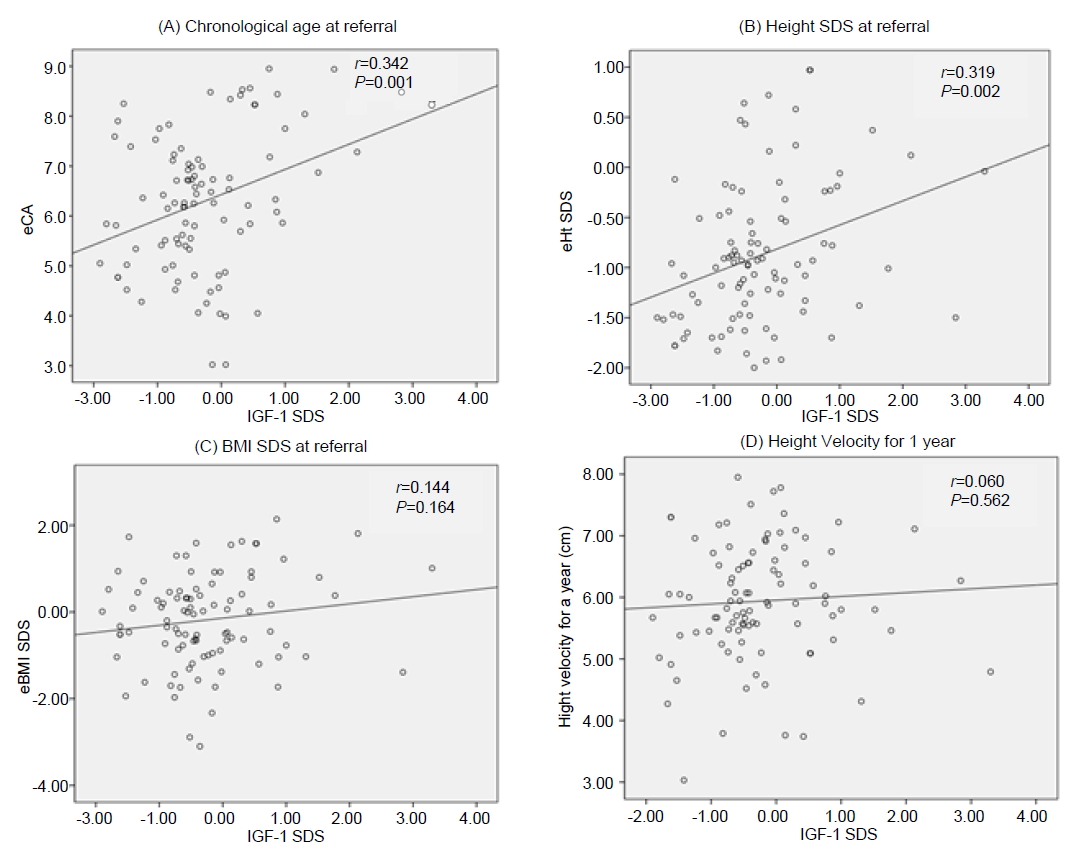
2. WHO Multicentre Growth Reference Study Group. WHO Child Growth Standards based on length/height, weight and age. Acta Paediatr Suppl 2006;450:76–85.


3. Stenhouse E, Wright DE, Hattersley AT, Millward A. The accuracy of birth weight. J Clin Nurs 2004;13:767–8.


4. Gluckman PD, Hanson M, Zimmet P, Forrester T. Losing the war against obesity: the need for a developmental perspective. Sci Transl Med 2011;3:93.

5. Dewey KG, Peerson JM, Brown KH, Krebs NF, Michaelsen KF, Persson LA, et al. Growth of breast-fed infants deviates from current reference data: a pooled analysis of US, Canadian, and European data sets. World Health Organization Working Group on Infant Growth. Pediatrics 1995;96(3 Pt 1):495–503.

8. Tanner JM. Fetus into man: physical growth from conception to maturity. Cambridge (MA): Harvard University Press. 1989.
9. Roemmich JN, Rogol AD. Exercise and growth hormone: does one affect the other? J Pediatr 1997;131(1 Pt 2):S75–80.


10. Wideman L, Weltman JY, Hartman ML, Veldhuis JD, Weltman A. Growth hormone release during acute and chronic aerobic and resistance exercise: recent findings. Sports Med 2002;32:987–1004.


11. Haspolat K, Ece A, Gürkan F, Atamer Y, Tutanç M, Yolbaş I. Relationships between leptin, insulin, IGF-1 and IGFBP-3 in children with energy malnutrition. Clin Biochem 2007;40:201–5.


12. He Q, Karlberg J. Bmi in childhood and its association with height gain, timing of puberty, and final height. Pediatr Res 2001;49:244–51.


13. Vignolo M, Naselli A, Di Battista E, Mostert M, Aicardi G. Growth and development in simple obesity. Eur J Pediatr 1988;147:242–4.


16. Ahmed SF, Farquharson C. The effect of GH and IGF1 on linear growth and skeletal development and their modulation by SOCS proteins. J Endocrinol 2010;206:249–59.


17. Moon JS, Lee SY, Nam CM, Choi JM, Choe BK, Seo JW, et al. 2007 Korean National Growth Charts: review of developmental process and an outlook. Korean J Pediatr 2008;51:1–25.

18. Marshall WA, Tanner JM. Growth and physiological development during adolescence. Annu Rev Med 1968;19:283–300.


19. Hyun SE, Lee BC, Suh BK, Chung SC, Ko CW, Kim HS, et al. Reference values for serum levels of insulin-like growth factor-I and insulin-like growth factor binding protein-3 in Korean children and adolescents. Clin Biochem 2012;45:16–21.


21. Alawneh H, Khaledi O, Maita J, Fugaha N. Insulin like growth factor 1 as an indicator of growth hormone deficiency. J Res Med Sci 2015;22:13–7.

23. Nilsson O, Marino R, De Luca F, Phillip M, Baron J. Endocrine regulation of the growth plate. Horm Res 2005;64:157–65.


24. Wang X, Xing KH, Qi J, Guan Y, Zhang J. Analysis of the relationship of insulin-like growth factor-1 to the growth velocity and feeding of healthy infants. Growth Horm IGF Res 2013;23:215–9.


25. Yackobovitch-Gavan M, Lebenthal Y, Lazar L, Shalitin S, Demol S, Tenenbaum A. Effect of nutritional supplementation on growth in short and lean prepubertal children after 1 year of intervention. J Pediatr 2016;179:154. –9. e1.


26. WOLFF OH. Obesity in childhood; a study of the birth weight, the height, and the onset of puberty. Q J Med 1955;24:109–23.

27. Cheek DB, Schultz RB, Parra A, Reba RC. Overgrowth of lean and adipose tissues in adolescent obesity. Pediatr Res 1970;4:268–79.


29. Nam SY, Lee EJ, Kim KR, Cha BS, Song YD, Lim SK, et al. Effect of obesity on total and free insulin-like growth factor (IGF)-1, and their relationship to IGF-binding protein (BP)-1, IGFBP-2, IGFBP-3, insulin, and growth hormone. Int J Obes Relat Metab Disord 1997;21:355–9.