Leptin and adiponectin levels in girls with central precocious puberty before and during GnRH agonist treatment
Article information
Abstract
Purpose
The effects of gonadotropin-releasing hormone agonist (GnRHa) treatment on the energy metabolism in girls with central precocious puberty (CPP) are controversial. We focused the changes and related factors of serum levels of leptin and adiponectin in girls with CPP before and during GnRHa treatment.
Methods
Thirty girls with idiopathic CPP were enrolled in the study. Their auxological data and fasting blood were collected at the baseline and after six months of GnRHa treatment.
Results
After treatment, height (P<0.001), weight (P<0.001), and serum leptin levels (P=0.033) were significantly increased, whereas body mass index (BMI), homeostasis model of assessment-insulin resistance, serum adiponectin levels, and adiponectin/leptin ratio exhibited no significant changes. A Pearson correlation analysis showed that height, weight, BMI, and their standard deviation scores (SDSs), but not basal LH, FSH, and estradiol, were significantly correlated with serum leptin levels before and after GnRHa treatment. After a multiple linear regression analysis, only BMI was associated with serum leptin levels. Moreover, leptin SDSs adjusted for BMI were not significantly different before and after GnRHa. The Δ leptin levels (r2=0.207, P=0.012), but not with Δ leptin SDS (r2=0.019, P=0.556), during GnRHa treatment were positively correlated with Δ BMI.
Conclusion
These results suggest that GnRHa treatment in girls with CPP does not affect serum levels of leptin and adiponectin and insulin resistance. Serum leptin levels were depend on the changes in BMI during GnRHa treatment.
Introduction
Central precocious puberty (CPP) is a common and rising problem in children1). The cause of CPP is mostly idiopathic in girls1). However, CPP occurs at a high rate among children with obesity, and it has been shown that pubertal growth is dependent on the quantity and distribution of body fat2). Some studies have reported an effect of body mass index (BMI) on abnormal sexual maturation in children with CPP3). The gonadotropin-releasing hormone agonist (GnRHa) is the standard for the treatment of CPP with progressive puberty and accelerative growth1). Although the efficacy and safety of GnRHa treatment for CPP have been well described, the data available in the literature regarding the effects of GnRHa treatment on overweight status or obesity in children with CPP are controversial145).
Obesity is a condition that is characterized by the accumulation of excess adipose tissue in the body. Leptin, which was the first adipokine to be identified, plays a role in the regulation of body fat mass through appetite control and increased metabolism6). The production of leptin show positive correlation with adipose tissue mass and food intake6). Adiponectin is an adipocyte-derived hormone that improves insulin sensitivity in the liver and skeletal muscle7). Adiponectin gene expression and blood levels are negatively correlated with BMI7). Central adiponectin/leptin signaling may represent a physiological pathway that is associated with energy metabolism8). Adioposity and nutritional state are well-known factors for timing of sexual maturation. Adipokines are an important link between adioposity, nutritional state, and puberty, because these hormones are secreted in adiposity and transfer the status of body energy reserves to the brain. In fact, patients with leptin deficincy or severe anorexia nervosa develop hypogonadotropic hypogonadism9).
Recently, the modulation of the hypothalamus-pituitary axis by leptin was shown to perform an essential role in the control of female puberty10). And some studies show that the serum leptin levels in girls with CPP are higher than those of normal pubertal controls, but is not significantly different from those in girls with premature thelarche11). However, it is not fully understood whether adipokines, such as leptin and adiponectin, are related with early acceleration of sexual development in children.
In this study, we studied to evaluate the relationship between serum leptin and adiponectin levels, metabolic components, and sexual hormones in girls with CPP before and during GnRHa treatment, and to determine the changes of serum leptin and adiponectin levels and insulin resistance after GnRHa treatment.
Materials and methods
1. Subjects
This study protocols were performed after approval by the Institutional Review Board of the Chungnam National University Hospital, Daejeon, South Korea, and written informed consent was obtained from patient participants and their legitimate guardians (approval number: 2014-10-009).
A total of 30 girls diagnosed with idiopathic CPP who had received 6 injections (every 4 weeks) of GnRHa (50–100 µg/kg, leuprolide acetate; Takeda, Osaka, Japan) were recruited among the children who visited our outpatient growth clinic at the Department of Pediatrics, Chungnam National University Hospital, between January 2012 and July 2013. CPP was defined as breast budding as a first sign of puberty before 8 years of age, accompanied by a bone age advanced by at least 1 year compared with chronological age, and a peak luteinizing hormone (LH) level>5.0 IU/L in the course of a rapid-acting GnRH stimulation test (0.1-mg Relefact LHRH; Sanofi-Aventis, Frankfurt am Main, Germany). Girls with idiopathic CPP who had a history of medication associated with altered pubertal timing or gonadal development, such as steroids, growth hormone, or with pathological CPP who were found to have abnormalities on brain MRI or pelvis sonography or genetic diseases were excluded from the study.
2. Auxological and clinical data
Information on age, height, weight, parental height, pubertal status, bone age, and past medical history was collected from the medical records of the patients. The standard deviation scores (SDSs) of height, weight, and BMI were calculated using the 2007 growth reference for Korean children and adolescents12). Bone age was determined using Greulich and Pyle atlas. Predicted adult height was calcuated according to the Bayley-Pinneau method. The homeostasis model assessment of insulin resistance (HOMA-IR) was calculated as fasting insulin concentration (mU/L)×fasting glucose concentration (mmol/L)/22.5.
3. Blood sampling
Basal fasting blood samples before GnRH injection were drawn from the antecubital vein in all patients. Poststimulation samples (for the measurement of LH and follicle-stimulating hormone (FSH) levels) were taken 15, 30, 45, 60, and 90 minutes after injection. Posttreatment venous fasting blood samples were collected 30 minutes after 6 injections of GnRHa. Serum samples were obtained by centrifuging blood samples (2,000 rpm, 4℃, 10 minutes) within 30 minutes of sampling; samples were immediately stored at –75℃ until analysis.
4. Laboratory measurements
Serum LH and FSH levels were measured using a radioimmunoassay (BioSource SA, Nivelles, Belgium). Serum estradiol level was measured using a chemiluminescence immunoassay kit (Estradiol II, Roche Diagnostics, Indianapolis, IN, USA) and a Roche Analytics E-170 Immunology Analyzer (Diamond Diagnostics, Holliston, MA, USA). Progesterone level was measured using a radioimmunoassay kit (Progesterone, Siemens Healthcare Diagnostics, East Walpole, MA, USA) and an ADVIA Centaur CP Immunoassay System (Siemens, Seoul, Korea). Serum leptin level was measured using the human leptin “Dual Range” enzyme-linked immunosorbent assay (ELISA) kit (EZHL-80SK, Merck Millipore Co., Darmstadt, Germany) with a sensitivity of 0.5 ng/mL, an interassay coefficient of variation (CV) of 2.6%–6.2%, and an intra-assay CV of 2.6%–4.6%. Finally, adiponectin level was measured using the human adiponectin ELISA kit (EZHADP-61K, Merck Millipore Co.) with a sensitivity of 1.5 ng/mL, an interassay CV of 2.4%–8.4%, and intra-assay CV of 1.0%–7.4%, on a SpectraMax 190 Microplate Spectrophotometer (Molecular Devices, Sunnyvale, CA, USA).
5. Leptin SDS adjusted BMI
The calculation of leptin SDS adjusted for BMI was based on the equations provided by Blum et al.13).
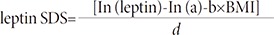
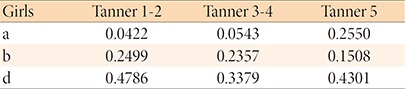
For example, the leptin SDS of a girl with breast budding, BMI = 16.7 kg/m2, and 2.45 ng/mL of serum leptin level, is leptin SDS = [In (2.45) – In (0.0422) – 0.2499×16.7]/0.4786 = –0.23.
6. Statistical analysis
The numerical data are presented as the mean±standard deviation. Paired-samples t-tests were used to compare the means of auxological and clinical laboratory data, including serum leptin and adiponectin levels at the baseline and after six months of GnRHa treatment. Bivariate and partial correlate analyses were used to determine the relationship between serum leptin or adiponectin levels and anthropometric, metabolic, and hormonal parameters. A stepwise multiple linear regression model was developed to evaluate the independent factors that were associated with serum leptin levels and other variables. A paired sample t-test was used to compare the mean of leptin SDS according to GnRHa treatment. To determine the effect of BMI on serum leptin levels in girls with CPP during GnRHa treatment, the relationship between the changes of leptin levels or leptin SDS values and the changes in BMI were presented as regression linear graphs, which were prepared using GraphPad Prism 5 for Windows (GraphPad Software Inc., San Diego, CA, USA). Data analyses were conducted using IBM SPSS Statistics ver. 20.0 (IBM Co., Armonk, NY, USA), and P<0.05 was considered significant.
Results
1. Auxological and laboratory characteristics of the participants
The anthropometric and laboratory characteristics of our subjects with CPP are summarized in Table 1. At the baseline, the chronological and bone age of girls with CPP was 8.1±0.7 and 10.2±0.9 years, respectively. After 6 months of GnRHa treatment, their bone age (P<0.001), height (P<0.001), and weight (P<0.001) were significantly increased, and the serum levels of basal LH (P=0.003), basal FSH (P=0.002), and estradiol (P=0.001) were decreased. Metabolic parameters, such as fasting glucose and insulin, HOMA-IR, serum adiponectin levels, and serum adiponectin/leptin ratio, were not significantly increased during GnRHa treatment. Only serum leptin levels were significantly increased in girls with CPP during GnRHa treatment (5.00±3.83 ng/mL→6.63±4.87 ng/mL, P=0.033).
2. Correlation between serum leptin and adiponectin levels and other variables at the baseline and after 6 months of GnRHa treatment
Serum adiponectin level was only negatively associated with weight (r=–0.428, P=0.018) after 6 months of GnRHa treatment. The serum leptin levels in girls with CPP were positively associated with height, weight, weight SDS, BMI, and BMI SDS. In particular, weight (r=0. 693, P<0.001; r=0.777, P<0.001) and BMI (r=0.626, P<0.001; r=0.752, P<0.001) exhibited strong positive correlations with serum leptin levels at the baseline and after 6 months, respectively. The serum levels of glucose and insulin and HOMA-IR were significantly correlated with serum leptin levels at the baseline, and height SDS was correlated with the serum levels of leptin only after 6 months. Serum adiponectin/leptin ratios showed inversely significant correlation with weight, BMI, and BMI SDS at the baseline and after six months of GnRHa treatment, and with weight SDS after 6 months of GnRHa treatment (Table 2).
3. The effect of BMI on serum leptin levels before and during GnRHa treatment
A stepwise multiple regression analysis was performed to determine the independent factors that affected serum leptin levels among height, weight, weight SDS, BMI, BMI SDS, fasting glucose and insulin levels, and HOMA-IR (Table 3). Among them, only BMI significantly predicted serum leptin levels in girls with CPP, at the baseline (F1, 28=18.065, P<0.001, R2=0.370) and after 6 months of GnRHa treatment (F1, 22=40.636, P<0.001, R2=0.633).
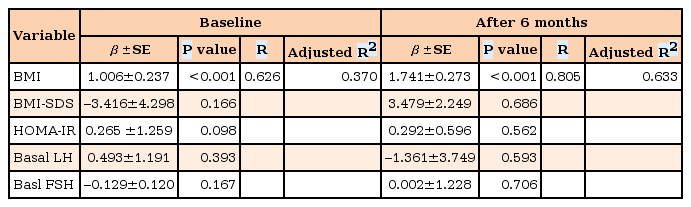
Stepwise multiple linear regression analysis between serum leptin levels and anthropometric, hormonal, and metabolic parameters in girls with central precocious puberty before and during GnRHa therapy
Using Blum's equation of leptin SDS, we converted the serum leptin levels to leptin SDS adjusted for BMI. The leptin SDS values were not significantly increased after 6 months of GnRHa treatment (0.065±1.318→0.398±1.249, P=0.234) (Table 1). Moreover, the changes in serum leptin levels (r2=0.207, P=0.012) (Fig. 1A) during GnRHa treatment exhibited positive correlations with the changes in BMI, but the changes in leptin SDS (r2=0.019, P=0.556) (Fig. 1B) did not.
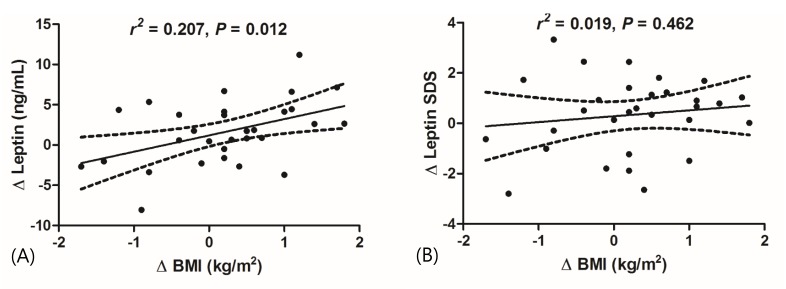
Correlation between changes in serum leptin levels and body mass index (BMI) in girls with central precocious puberty during GnRH agonist treatment. (A) Changes in serum leptin levels (r2=0.207, P=0.012), but not (B) changes in leptin SDS (r2=0.019, P=0.462), were positively correlated with changes in BMI. The linear regression is presented as an interpolate slope (solid line) and 95% confidence limits (dotted lines).
Discussion
The major findings of this study were: (1) changes in leptin levels in girls with CPP during treatment with GnRHa were associated only with changes in BMI, and not with puberty states and suppression of the hypothalamus-pituitary-gonad (H-P-G) axis; and (2) glucose homeostasis and serum adiponectin levels in girls with CPP were not changed duting GnRHa treatment.
There is a strong tendency for girls with CPP to be overweight /obese at diagnosis4). There are also concerns regarding whether the administration of GnRHa to girls with CPP may affect their weight status and metabolism4). It is not clear why children with CPP are more obese than those with normal puberty. Moreover, the definite effects of GnRHa on weight remain unknown. However, it is supposed that the hormonal changes of puberty itself trigger an increase in BMI, or that a higher BMI contributes to the earlier onset of puberty14).
Leptin may serve as a circulating signal of nutritional or adipose status. Nutritional distress associated with energy excess, as well as energy insufficiency, can influence the onset of puberty and interfere with normal estrogen cycles by altering endocrine function15). However, the precise role of leptin in the onset of puberty and in the H-P-G axis remains uncertain. Leptin does not apparently serve as a triggering signal, but acts mainly as a permissive signal that permits puberty to occur16). Recently, some reports have focused on the role of the relationships between leptin, kisspeptin, and the H-P-G axis as a metabolic control of puberty initiation and progression9). In adult women, suppression of the H-P-G axis by GnRHa does not affect serum leptin levels17). Su et al.18) demonstrated that the administration of GnRHa treatment to girls with CPP does not affect serum leptin levels, and that serum leptin levels are merely associated with weight and BMI. Moreover, serum leptin levels in girls with CPP were not significantly different from those detected in Tanner-matched healthy girls, but were associated with BMI in girls with and without CPP19). These data are similar to our findings. The serum leptin levels detected here in girls with CPP were not associated with peak LH and FSH levels, as assessed using a GnRH stimulation test (data not shown), or with basal LH, FSH, and estradiol levels before and after GnRHa treatment. Moreover, serum leptin levels were increased after GnRHa treatment, however these findings may have resulted from an increase in BMI itself after using a multiple linear regression analysis. The results reported above suggest that circulating leptin levels do not indicate or determine H-P-G status and are not directly linked with gonadotropins and sex hormones. Unfortunately, this study did not include healthy girls with normal puberty as controls, and did not analyze the differences in serum levels in girls according to early sexual maturation.
Changes in body composition and glucose homeostasis are closely linked to sexual maturation, and the total body fat percentage increases in girls during puberty20). Several studies have demonstrated that girls with CPP have significantly increased total body fat and show decreased insulin sensitivity at initial diagnosis compared with those with normal puberty212223). Early alterations in obesity-associated adipose tissue biology in childhood have been linked to leptin and insulin resistance24). Taşcilar et al.5) described an elevation in trunk fat mass and HOMA-IR in girls with CPP treated with GnRHa. However, Sørensen et al.25) reported that metabolic alterations in girls with CPP persisted beyond the withdrawal of sex steroids by GnRHa treatment. Adiponectin plays an important role in the maintenance of energy homeostasis, via mitochondrial biogenesis and oxidative metabolism in skeletal muscle and by inhibiting lipolysis in adipocytes7). Moreover, Inoue et al.26) reported that the serum adiponectin/leptin ratio is a more effective marker of insulin resistance than HOMA-IR in subjects without hyperglycemia. Moreover, serum adiponectin levels decrease significantly during pubertal development in boys, but not in girls, and exhibit a negative correlation with testosterone, but no association with estradiol, in healthy children27).
To the best of our knowledge, the exact mechanism underlying the changes in adiponectin and glucose homeostasis in girls with CPP according to GnRHa treatment has not been elucidated. Studies performed in adult women with polycystic ovarian syndrome have demonstrated that adiponectin may play a role in linking adiposity with insulin resistance28). Other adult-based studies reported that adiponectin levels were increased in response to GnRHa-induced ovarian suppression29), but decreased by transdermal estrogen30).
In this study, the levels of glucose, insulin, and HOMA-IR did not exhibit the statistically significant tendency to increase after 6 months of GnRHa treatment. In addition, serum adiponectin levels and adiponectin/leptin ratio were not significantly increased after GnRHa treatment. Moreover, we found no association between serum adiponectin levels and LH, FSH, estradiol, glucose, and insulin levels at the baseline and after 6 months of GnRHa treatment. Our results suggest that the administration of GnRHa to girls with CPP may not affect their insulin resistance and fat deposit during treatment, and that the circulating adiponectin levels in girls with CPP may not be correlated with the H-P-G axis.
This study had several limitations. We did not examine longitudinally the changes in leptin and adiponectin levels or in body composition after discontinuation of the GnRHa treatment. Moreover, we did not consider other metabolic risk factors, such as food intake, physical activity, and serum lipid profiles. We used a method of HOMA-IR, not hyperinsulinemic euglycemic clamp, for insulin resistance. Finally, we did not assess the variables in normal controls and our sample size was small.
In conclusion, GnRHa treatment in girls with CPP did not affect the levels of serum leptin and adiponectin or insulin resistance. The serum leptin levels in girls with CPP were associated with changes in BMI, similar to what was observed in controls with normal puberty. Further longitudinal studies using larger samples are needed to define the role of leptin and adiponectin in pubertal development.
Acknowledgments
This study was financially supported by a research fund from Chungnam National University in 2012 (#2012-1915).
Notes
Conflict of interest: No potential conflict of interest relevant to this article was reported.


