Introduction
In children, adolescents, and adults, obesity causes not only excessive fat accumulation in the human body, but also dyslipidemia and hypertension. In addition, obesity increases insulin resistance over time, causing glucose intolerance and metabolic disorders such as type 2 diabetes mellitus (T2DM) [
1].
In many cases of T2DM, glucose intolerance appears early in the course of the disease and gradually progresses thereafter. Thus, glucose intolerance is a prognostic factor for diabetes [
2] and cardiovascular disease [
3]. Insulin resistance is the main cause of cardiovascular diseases. Insulin has a complex effect on blood vessels. When insulin is present in the blood in normal concentration, the vasoprotective action is dominant; however, in the insulin-resistant state, the nitrate oxide (NO) pathway activated by insulin is damaged, and the mitogen-activated protein kinase (MAPK) pathway becomes predominant, resulting in blood vessel constriction and inflammatory reactions, which increase blood pressure and cause inflammation [
4]. Lifestyle corrections can prevent or delay the progression of glucose intolerance to diabetes.
The rates of obesity in children and adolescents have nearly doubled worldwide in recent decades [
5], and several studies have reported that the prevalence of T2DM has recently increased in children and adolescents, especially those with obesity. An increasing trend of metabolic abnormalities, such as T2DM, obesity, dyslipidemia, and hypertension, in children and adolescents has been reported in the United States, Europe, and Japan [
6].
In England, Sinha et al. [
7] conducted oral glucose tolerance tests on adolescents and children, and the results showed that impaired glucose tolerance is highly prevalent among children and adolescents with severe obesity.
In the United States, Li et al. [
8] analyzed the relationship between glucose intolerance and metabolic risk factors in adolescents aged 12ŌĆō19 years using data from the National Health and Nutrition Examination Survey. Prediabetes was highly prevalent among adolescents with obesity and demonstrated increased metabolic risk in this population.
In Korea, there have been some studies on the association between obesity and T2DM in children and adolescents; however, no studies have evaluated the prevalence of fasting hyperglycemia and the relationships between fasting hyperglycemia and metabolic indicators [
9,
10].
In this study, to assess the risk of diabetes in domestic children and adolescents, the prevalence of fasting hyperglycemia was calculated, and the trend was evaluated using multi-year data. To verify this, we planned to evaluate diabetes risk based on fasting blood sugar level in Korean teenagers using Korea National Health and Nutrition Examination Survey (KNHANES) data from the past decade. Body mass index (BMI), blood pressure, and cholesterol and triglyceride levels were measured to assess the relationships between fasting hyperglycemia and metabolic indicators based on the KNHANES data.
Discussion
This study revealed an increasing trend in the prevalence of fasting hyperglycemia, including IFG and DMFBG, in Korean teenagers from 2007 to 2018. The prevalence of IFG and DMFBG in Korean teenagers increased significantly from 4.76% in the fourth KNHANES (2007ŌĆō2009) to 11.36% in the seventh KNHANES (2016ŌĆō2018).
The prevalence of IFG and DMFBG increased from 4.79% in the fifth KNHANES (2010ŌĆō2012) to 10.03% in the sixth KNHANES (2013ŌĆō2015). We confirmed that there were no changes in the blood glucose measurement equipment or reagents at that time. In addition, it was confirmed that the scale calculated by prevalence in this study was similar to that of the internal KNHANES data. Therefore, it was difficult to clearly determine whether a change in the period was actually a change or whether a systematic error was involved.
The increasing prevalence of fasting hyperglycemia is consistent with that of recent decades, showing an increasing prevalence of fasting hyperglycemia among children and adolescents in industrialized countries, such as the United States and the United Kingdom [
7,
8]. Several studies conducted in the United States reported that the prevalence of pediatric diabetes patients was less than 4% before the 1990s but had increased up to 45% in recent studies. This trend is not limited to the United States. In a study on the prevalence of type 2 diabetes in middle and high school students in Tokyo, a urine glucose screening test was performed, and a glucose tolerance test was performed as a confirmatory test. Our results show that the diabetes prevalence rate from 1976 to 1980 was 7.3/10,000 people, increasing to 13.9/10,000 from 1991 to 1995 [
15].
According to a survey by Li et al. [
8], the prevalence of IFG, impaired glucose intolerance, and prediabetes was 13.1%, 3.4%, and 16.1%, respectively, in adolescents aged 12ŌĆō19 years in the United States. Prediabetes was defined as IFG (fasting glucose > 100 mg/dL and <125 mg/dL) and/or impaired glucose intolerance (2 hours after oral glucose tolerance test, glucose > 140 mg/dL and < 200 mg/dL). Metabolic risk factors were classified into 4 factors: central obesity, blood pressure, triglycerides, and HDL cholesterol. The results showed that metabolic risk factors were significantly associated with prediabetes but not with body weight.
The causes of impaired glucose tolerance impacted by obesity include hyperinsulinemia and insulin resistance [
16]. As adipocytes proliferate in individuals with obesity, serum levels of hormones, nonesterified fatty acids, glycerol, and leptin secreted by adipose tissue, as well as C-reactive protein, tumor necrosis factor-╬▒, angiotensinogen, fibrinogen, interleukin, cortisol, and plasminogen activator inhibitor-1 increase. Adiponectin concentration decreases, resulting in a decreased sensitivity of cells to insulin. As the insulin responsiveness of the cells decreases, the serum concentration of insulin becomes consistently high; that is, hyperinsulinemia is induced and, as hyperinsulinemia continues, dysfunction of pancreatic islet ╬▓-cells that secrete insulin is accompanied by progression to T2DM [
17-
19].
The fasting hyperglycemia group showed an increase in metabolic indicators of blood pressure, triglycerides, and cholesterol levels compared to the normal fasting blood glucose group. The causes of hypertension and metabolic abnormalities involving glucose intolerance are the result of insulin resistance. Insulin is an anabolic hormone that plays an important role in the storage of sugar, fat, and energy and contributes to sodium homeostasis by promoting sodium reabsorption in the kidneys, as well as having metabolic effects. In obesity, hyperinsulinemia occurs as a result of insulin resistance, activating sympathetic nerves, increasing sodium reabsorption in the kidneys, and increasing blood pressure [
20].
Insulin has a complex effect on the blood vessels. It stimulates the production of NO in endothelial cells, induces vasodilation and anti-inflammatory reactions to protect blood vessels, and concurrently promotes the MAPK pathway, leading to vasoconstriction and inflammation. The blood vessel-destructive action that triggers the reaction occurs simultaneously. When insulin blood concentration is normal, the vasoprotective action is dominant; however, in the insulin-resistant state, the NO pathway activated by insulin is disrupted, and the MAPK pathway becomes predominant, resulting in blood vessel constriction and inflammatory reactions, which increase blood pressure and cause inflammation [
21-
24].
We demonstrated that the increasing rate of fasting hyperglycemia was higher in boys than in girls. The posited reason for this was the difference in insulin resistance due to gender differences between growth and sex hormones. During puberty, growth hormone and insulin-like growth factor (IGF)-1 increase rapidly and subsequently decrease after puberty is complete. Growth hormone and IGF-1 affect insulin resistance during normal pubertal development. On average, girls start puberty at 10ŌĆō11 years of age and end at 15ŌĆō17 years. Boys start puberty at 11ŌĆō12 years of age and end at 16ŌĆō17 years. This study targeted children and adolescents between the ages of 10 and 18 and suggests that boys have higher fasting blood glucose level than girls because the proportion of boys undergoing puberty with increased insulin resistance is relatively higher than that of girls [
25].
According to our results, during the 2013ŌĆō2018 period, the average age of the normal blood glucose group was 14.46 years, whereas that of the diabetes high-risk group was 13.75 years. Fasting blood glucose level was significantly higher in the younger patients. The incidence of T2DM is more higher during puberty, when explosive increases in growth and sex hormones occur. This increase in growth hormone secretion causes a temporary period of insulin resistance [
26]. In our study, in participants aged 16ŌĆō18 years, it was estimated that the effects of growth and sex hormones had decreased, and blood glucose had declined.
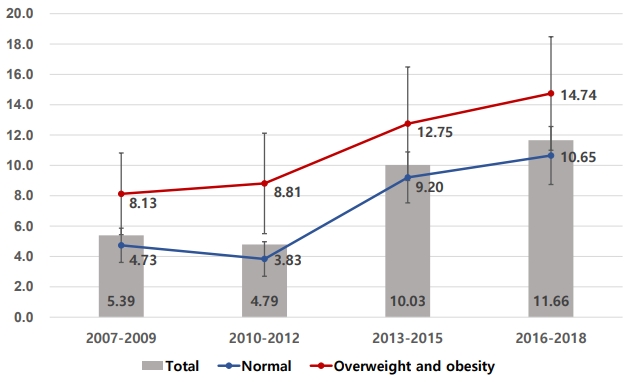
As shown in
Fig. 1, fasting blood glucose level was higher in the overweight and obesity groups (Ōēź85th percentile or BMI Ōēź 25.0 kg/m
2) than in the normal weight group. However, as in the overweight and obesity groups, fasting blood glucose had increased in the last decade in a similar pattern in the normal weight group.
Parent diabetes history was significantly higher in the diabetes high-risk group (12.74%) than in the normal blood glucose group (6.20%), and there was no significant difference in total household income, total energy intake, or intake of macronutrients. It is well known that a family history of T2DM is a risk factor for diabetes. However, it is controversial whether environmental or genetic factors are the main risk factors. Many studies have shown that environmental factors, such as physical activity and BMI, together with familial characteristics and genetic factors, exert a combined, simultaneous effect on diabetes risk [
27].
In terms of nutritional composition, the total energy intake, excess calorie intake, and fat and carbohydrate intake were not significantly different between the normal blood glucose and hyperglycemia groups. The results of one diabetes study in domestic adults showed that the diabetes group had a higher percentage of energy intake from carbohydrates than the normal blood glucose group [
25]. Given that carbohydrates are broken down into monosaccharides in the blood, it is easy to deduce that the higher is the intake of carbohydrates, especially carbohydrates with a high glycemic index, the higher will be the blood sugar level after a meal [
26]. However, in this study, there was no difference between carbohydrates or other macronutrients in the nutritional compositions of the normal and hyperglycemic groups.
Since this study was based on KNHANES data, nutritional details such as glycemic index were not considered, and environmental factors such as exercise habits were not investigated. Therefore, it is difficult to determine the cause of the recent increase in blood sugar level. This is a limitation of this study, and further research is required. Another weakness is that serum insulin was not directly measured or directly inferred from serum glucose status, although the main pathogenesis of diabetes is hyperinsulinemia. The strength of this study is that it evaluated trends using multi-year data at the national level, and the reliability of the data is high because it was collected from more than 5,000 people over a long period of time, about 10 years.
In conclusion, the proportion of fasting hyperglycemia among adolescents has increased over the past decade and is believed to be associated with an increase in metabolic abnormalities such as hypertension and hypertriglyceridemia. A more detailed study is necessary to evaluate the effects of environmental factors and eating habits; nevertheless, obesity, as per consensus, increases the risk of metabolic syndromes such as diabetes, hypertension, and dyslipidemia. Prevention of the progression of prediabetes in adolescents to diabetes in adulthood has recently become an urgent issue in Korea.