 |
 |
- Search
| Ann Pediatr Endocrinol Metab > Volume 25(4); 2020 > Article |
|
Abstract
The Committee on Dyslipidemia of Korean Pediatric and Adolescents of the Korean Society of Pediatric Endocrinology has newly developed evidence-based clinical practice guidelines for dyslipidemia in Korean children and adolescents. These guidelines were formulated with the Grading of Recommendations, which include both the strength of recommendations and the quality of evidence. In the absence of sufficient evidence, conclusions were based on expert opinion. These guidelines are based on the 2011 National Heart, Lung, and Blood Institute Guidelines, which focus on the prevention of cardiovascular disease in children and draw from a comprehensive review of evidence. These guidelines contain the definition of and screening process for dyslipidemia and introduce new dietary methods: the Cardiovascular Health Integrated Lifestyle Diet (CHILD)-1, the CHILD-2-low-density lipoprotein cholesterol, and the CHILD-2-triglyceride. Potential drug therapies for dyslipidemia along with their main effects and doses were also included.
Cardiovascular disease (CVD) is a major cause of morbidity and mortality worldwide, including in Korea [1,2]. The prevalence of CVD in Korea has historically been much lower than the rate reported in Western countries. Recently, however, the CVD-associated mortality rate in Korea has increased to 27.6%, which is comparable to that in the United States (US) [2].
Increased total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), triglyceride (TG), and decreased high-density lipoprotein cholesterol (HDL-C) are well-known risk factors associated with CVD [3-6]. The Korean Heart Study, a 10-year prospective study of 430,920 adult men and women, also concluded that high TC and low HDL-C levels increased the risk of CVD [7].
Dyslipidemia is closely related to other CVD risk factors, such as hypertension, obesity, and smoking status, not only in adults but also in children and adolescents [3,8]. Furthermore, the prevalence of obesity and metabolic syndrome, which are other risk factors for CVD, has steadily increased in Korea and is now comparable to the rate reported in the US [6,9]. This trend likely resulted from recent changes in dietary habits among Korean people that are commonly associated with a western lifestyle [9,10].
Although CVD does not usually develop until the fourth decade of life, it is well known that atherosclerosis begins in childhood [8,11,12]. The initial stages of atherosclerosis and its progression are associated with dyslipidemia [13]. Autopsy data from the Pathobiological Determinants of Atherosclerosis in Youth Study showed a strong correlation between high cholesterol and the development of fatty streaks and fibrous plaques in coronary vasculature at an early age [14]. In the Bogalusa Heart Study, the extent of fatty streaks and fibrous plaques increased with age and was correlated with antecedent serum TG, very-high LDL-C, and obesity [3].
Among Korean children and adolescents aged 10ŌĆō19 years, 20% had at least one type of dyslipidemia [15]. The prevalence of hypercholesterolemia, high LDL-C, high TG, and low HDL-C concentrations among Korean children and adolescents was 6.5%, 4.7%, 10.1%, and 11.9%, respectively [16]. The rate of dyslipidemia in Korean obese adolescents has been reported as 56.1% [15].
Early recognition of and intervention for dyslipidemia in Korean children and adolescents is important for preventing CVD later in life. In this context, the Committee on Pediatric Dyslipidemia of the Korean Society of Pediatric Endocrinology (KSPE) has recently developed evidence-based clinical practice guidelines to treat dyslipidemia in Korean children and adolescents.
Several studies, such as the Lipid Research Clinics Prevalence Study and the National Health and Nutrition Examination Survey, have shown that there are sexual, racial, and ethnic differences in lipid profiles and also in the overall prevalence of dyslipidemia [17,18]. The mean and 50th percentiles for TC, LDL-C, non-HDL-C, TG, and HDL-C were similar between Koreans and Caucasians from the US [17,18]. According to the Korea National Health and Nutrition Examination Survey IV (2007ŌĆō2009), the 95th percentiles for TC and LDL-C and the 10th percentile for HDL-C in Korean children and adolescents aged 10ŌĆō18.9 years were 203 mg/dL, 129 mg/dL, and 38 mg/dL, respectively. The 90th and 95th percentiles for TG concentrations were 150 and 185 mg/dL, respectively. In addition, a non-HDL-C of 145 mg/dL corresponds to approximately the 90th to 95th percentiles [15,19].
The Committee of Clinical Practice Guidelines of the KSPE decided to adopt the National Heart Lung and Blood Institute (NHBLI) 2011 guidelines for dyslipidemia to prevent adulthood CVD by lifestyle modification or medical intervention [20]. The cutoff levels for serum lipid levels to diagnose dyslipidemia in Korean children and adolescents are listed and defined in Table 1.
There have been controversies in the optimum TG cutoff level. Because carbohydrates make up a major part of the Korean traditional diet, some researchers have insisted that a higher TG concentration of 150 mg/dL, which corresponds to the 90th percentile of Korean adolescents, is more appropriate in defining dyslipidemia [15]. However, recent studies have shown that the consumption of simple sugars, fructose, and alcohol is a main factor that increases TG levels [21,22]. Furthermore, because TG is the main target of lifestyle modification in dyslipidemia, we decided to define hypertriglyceridemia as a TG concentration > 130 mg/dL. For Korean children younger than 10 years of age, further studies will be required to determine more appropriate reference and cutoff points for dyslipidemia.
According to the cutoff points of the NHBLI guidelines, 19.7% of Korean children and adolescents from 10ŌĆō18 years of age had at least one abnormal lipid concentration [15]. The prevalence of hypercholesterolemia, high LDL-C, high TG, and low HDL-C (<40 mg/dL) was 6.5% (5.8% in males, 7.4% in females), 4.7% (4.1% in males, 5.5% in females), 10.1% (9.8% in males, 10.3% in females), and 11.9% (14.5% in males, 9.5% in females), respectively [15,16]. The prevalence of dyslipidemia was 20.7%, 39.6%, and 56.1% for boys (and 24.5%, 36.6%, and 53.1% for girls) who were normal weight, overweight, and obese, respectively [16].
Screening for lipids in children is based on the rationale that early identification and control of pediatric dyslipidemia will reduce the risk and severity of CVD in adulthood. Therefore, the Committee of Clinical Practice Guidelines of KSPE recommends "universal screening." Universal screening in this guideline might be performed to detect those with undiagnosed heterozygous familial hypercholesterolemia who would require more intensive treatment, possibly including pharmacological therapy [20,23]. In contrast, targeted screening based on family history of CVD or hypercholesterolemia fails to detect a substantial number (from 30%ŌĆō60%) of children with elevated lipid levels [24]. Almost 51% of untreated children with dyslipidemia will go on to develop clinical CVD by 50 years of age, and 5% do so by 30 years of age [25].
Universal lipid screening should be performed with the measurement of nonfasting non-HDL-C in all children aged 9ŌĆō11 and 17ŌĆō21 years. Non-HDL-C is calculated as follows: non-HDL-C=TCŌĆōHDL-C. Non-HDL-C includes all cholesterol types present in lipoprotein particles (LDL-C, lipoprotein(a), intermediate density lipoprotein, and very-low-density lipoprotein) that are considered atherogenic. Therefore, non-HDL-C is a better independent predictor of CVD than LDL-C and is also as good a predictor of future adulthood dyslipidemia that can replace LDL-C [26-28]. If non-HDL-C is >145 mg/dL, 2 additional fasting lipid panels (at least 2 weeks but no more than 12 weeks apart) should be obtained and the average values are used.
Screening for dyslipidemia is not recommended until 2 years of age. For children aged 2ŌĆō8 and 12ŌĆō16 years, routine lipid screening is also not recommended. Targeted screening is only encouraged if there is a family history of high cardiovascular risk or other risk factors and conditions. The risk factors and conditions to consider for the screening and treatment decisions in children with dyslipidemia are included in Tables 2 and 3 [20].
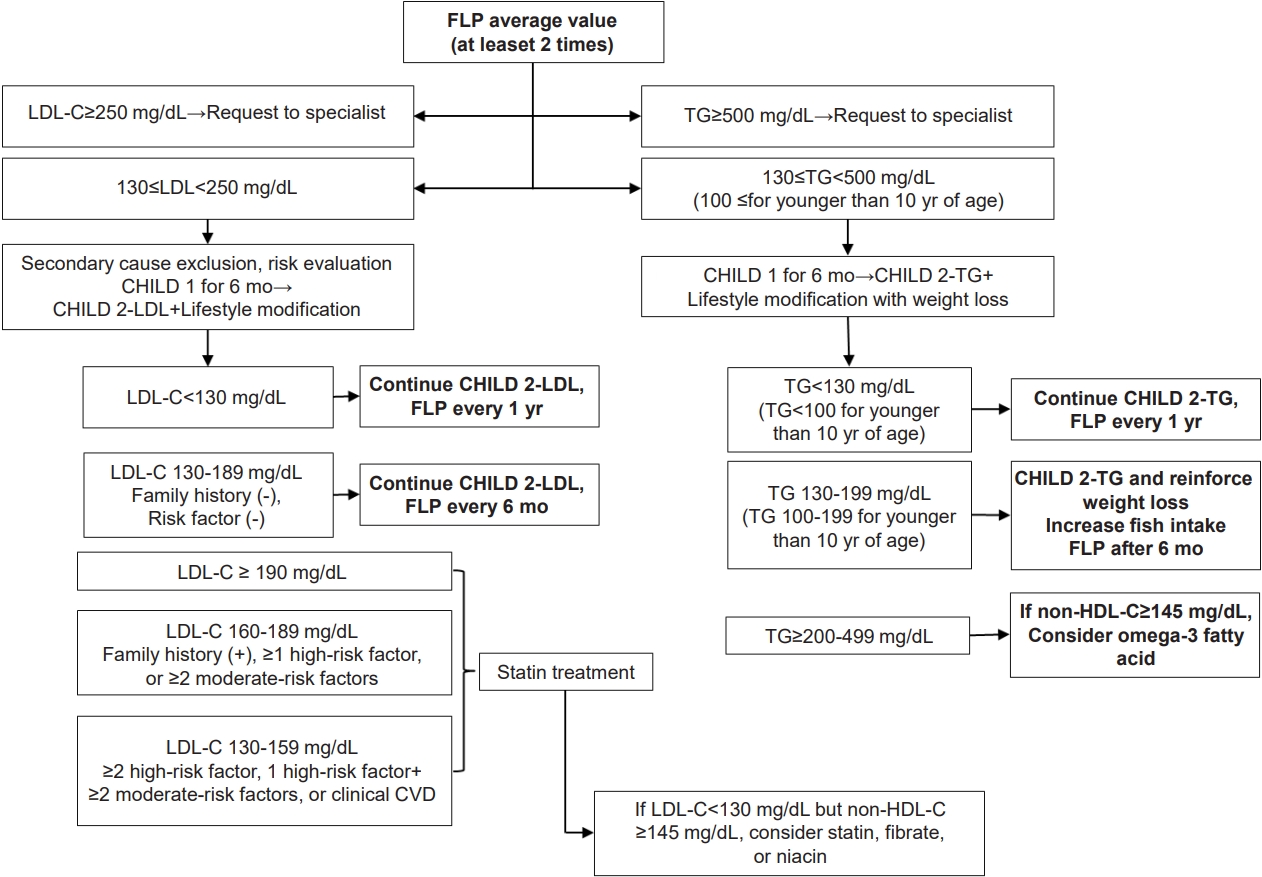
Rising rates of dyslipidemia in Korean children and adolescents might be due to increases in adoption of the western lifestyle, especially the westernized diet and decrease in physical activity [29,30]. Therefore, lifestyle modifications, such as promoting a healthy diet and regular physical activity, are thought to be essential in treating dyslipidemia. The algorithm used for dyslipidemia management is shown in Fig 1.
The KSPE guidelines recommend that all children and adolescents engage in moderate-to-vigorous physical activity for at least 1 hour a day and limit sedentary activity, including television, internet, and video games, to <2 hours a day. Cigarette exposure also should be strongly discouraged. In addition, all children and adolescents should try to attain an ideal body weight (body mass index Ōēż85th percentile for age and sex).
Any diet change must provide optimal nutrition for growth and development. The 2010 Dietary Guidelines for Americans recommend the Cardiovascular Health Integrated Lifestyle Diet (CHILD-1) be used in children and adolescents 2 years of age or older who are at risk for CVD [31].
Exclusively breastfeeding should be done until 6 months of age. If direct breastfeeding is impossible, use a breast pump, and if any breastfeeding is not available, iron-fortified infant formula should be fed. Breastfeeding should continue for at least 12 months while gradually adding solids. If breast milk decreases, feed iron-fortified formula until 12months. Infants under 12 months of age should not limit their fat intake without medical indication and limit 100% juice to about 120 mL per day, drink water without any other beverages.
Consult health-care provider about low-fat milk intake after 12 months of age if a family history of obesity, heart disease, or dyslipidemia is present [20].
If the fasting lipid profile findings exceed the therapeutic goals after a 3-month trial of the CHILD-1 diet, then the CHILD-2-LDL, or CHILD-2-TG diet should be recommended based on specific abnormal lipid parameters.
The detailed contents of each diet are listed in Table 4. The CHILD-1 urges specific protocols: drink fat-free unflavored milk primarily, limit/avoid sugar-sweetened beverages, encourage water consumption, limited total fat content (25%ŌĆō30% of the daily kcal/estimated energy requirements [EER] per day for age/gender), limit saturated fat 8%ŌĆō10% of the daily kcal/EER, avoid transfat as much as possible, and recommend monounsaturated and polyunsaturated fat up to 20% of the daily kcal/EER, and limit cholesterol intake to <300 mg/day [20]. In addition, these recommendations advocate the intake of dietary fiber (14 g/1,000 kcal), limiting naturally sweetened juice (no added sugar) to 120 mL/day, limiting sodium intake, and encouraging healthy eating habits overall. The CHILD-2 is divided into CHILD-2-LDL and CHILD-2-TG guidelines depending on the dyslipidemia target. Above all, it is important to refer these children and adolescents to a registered dietitian for family-based medical nutrition therapy and to decrease their total sugar intake. The CHILD-2 diet recommends the following: avoiding transfats, consuming <200 mg/day of cholesterol, and sustaining total, saturated, and monounsaturated fat at 25%ŌĆō30%, 7%, and <10% of total calories, respectively. Supportive dietary plans are different between the CHILD-2-LDL and the CHILD-2-TG. The CHILD-2-LDL diet recommends phytosterols up to 2 g per day as substitutes for dietary fats in children with familial hypercholesterolemia who are older than 2 years. Water-soluble fiber, such as psyllium, can be added to a low-fat, low-saturated fatty acid diet (6 g/day for children from 2ŌĆō12 years and 12 g/day for children >12 years). With the CHILD-2-TG, an increase in fish intake is critical in improving the levels of omega-3 fatty acids. TG levels are very responsive to weight loss, diet composition, and exercise [32].
Decisions regarding the need for drug therapy should be based on the average values from at least 2 fasting lipid tests obtained at least 2 weeks but no more than 3 months apart.
Drug therapy is recommended in children Ōēź10 years with a poor response to diet and lifestyle therapy for at least 6ŌĆō12 months [20]. The choice of medication depends on the lipid profile, age, sex, family history, and the pediatricians' experience [33]. In Korea, at least 0.41% of Korean children and adolescents are candidates for pharmacological treatment [16].
Children with average LDL-C levelsŌēź250 mg/dL or TGŌēź 500 mg/dL should be referred directly to a lipid specialist. If the TG concentration is >500 mg/dL, there is a risk of pancreatitis, and specialist consultation is therefore necessary [34].
Statin treatment is generally not started in children under the age of 10, and it is limited to patients homozygous for familial hypercholesterolemia or with LDL-CŌēź400 mg/dL, primary hypertriglyceridemiaŌēź500 mg/dL, CVD, or cardiac transplantation.
If the LDL-C remains Ōēź190 mg/dL after a 6-month trial of lifestyle management (CHILD-1ŌåÆCHILD-2-LDL) for children aged 10 years and older, statin therapy should be considered [20,35]. For patients with an LDL-C concentration between 160ŌĆō189 mg/dL, statin treatment should be considered if there is a positive family history of premature CVD in first-degree relatives, at least one high-level risk factor or risk condition, or at least 2 moderate-level risk factors or risk conditions. If the LDL-C concentration is between 130ŌĆō159 after 6 months of lifestyle modification, and if the children have at least 2 high-risk factors or conditions or at least one high-level risk factor and two or more moderate-risk factors or conditions or clinical CVD, statin therapy should be considered.
In children who are at least 10 years of age, the administration of statins, fibrates, or niacin may be considered if the LDL-C has reached its target but the non-HDL-C remains Ōēź145 mg/dL [20,36].
When TG improves with weight control and lifestyle changes in children with hypertriglyceridemia, medication therapy is unnecessary. As a supportive medical therapy, the use of omega-3 fatty acids is suggested along with lifestyle modification via the CHILD-2-TG, especially in children with hypertriglyceridemia. When the affected child has TG levels from 200ŌĆō499 mg/dL and a non-HDL-C result Ōēź 145 mg/dL, omega-3 fatty acid treatment may be considered [36].
However, safety issues remain unclear due to the limited experience with omega-3 fatty acids in children, which have only been used in a small number of cases [37]. (Table 5)
Statins and bile acid-binding resins are the two main classes of medications currently used to treat this condition in pediatric patients. Commonly used medications are listed in Table 6. Statins that are approved by the U.S. Food and Drug Administration (FDA) in children older than 10 years include pravastatin, simvastatin, lovastatin, and atorvastatin. Cholesterol-absorption inhibitors, such as niacin and fibrates, are not yet approved by the US FDA.
A statin is recommended as the initial medication of choice for children with elevated LDL-C or non-HDL-C levels. Statins inhibit the rate-limiting enzyme ╬▓-hydroxy ╬▓-methylglutaryl-CoA reductase and induce endogenous synthesis of cholesterol. Statins are effective at lowering cholesterol levels by 20%ŌĆō50% below the baseline. They are also known to have minimal side effects and do not affect growth [31,38,39]. The treatment regime should begin at the lowest dose given once daily at bedtime. Atorvastatin can be taken either in the morning or evening because of its long half-life. Before beginning statin treatment, baseline measurements of alanine aminotransferase, aspartate aminotransferase, and creatinine kinase should be obtained. Liver function tests, creatinine kinase, and a fasting lipid profile should be repeated at 4 and 8 weeks after the initiation of therapy and then again every 3 to 6 months. If liver enzymes are more than 3 times the upper limit or the creatinine kinase is >10 times the upper limit of the reference range, the statin medication should be stopped [40,41].
The target LDL-C value is typically <130 mg/dL, but if the patient is at high risk, such as with familial hypercholesterolemia or diabetes mellitus, the target LDL-C level should be maintained below 100 mg/dL. If target levels are not achieved within three months, the statin dose can be gradually increased to the maximum amount.
Since statins are potentially teratogenic, it is essential for physicians to confirm that adolescent girls are not pregnant before initiating statin therapy.
Bile acid sequestrants are the first line of therapy for children with dyslipidemia as these compounds are not absorbed systemically [42]. They work by preventing cholesterol reuptake in the enterohepatic circulation. However, cholestyramine and colestipol are unpalatable and are associated with gastrointestinal side effects, including bloating, nausea, diarrhea, and constipation. Therefore, compliance with this type of therapy is generally poor.
Ezetimibe (a cholesterol-absorption inhibitor) enters the enterohepatic circulation and reduces bile acid reuptake as well as cholesterol absorption. Ezetimibe is approved in children older than 10 years of age at a dosage of 10 mg/day as an adjuvant to statin therapy. In adults, ezetimibe has been shown to reduce the LDL-C by 20% [43]. No studies have investigated the treatment of ezetimibe alone in children, but data regarding children's experience with niacin and fibrates are also limited.
Therefore, niacin, fibrates, and ezetimibe should only be initiated after consulting with a lipid specialist. When these drugs are used in combination in children, they exert an increased combined effect, but the occurrence of side effects is not increased.
Atherosclerosis begins in childhood and can lead to CVD in adulthood. Early detection and proper management of dyslipidemia in children and adolescents are urgently needed to reduce adult CV morbidity. The 2017 Clinical Practice Guidelines for Dyslipidemia of Korean Children and Adolescents are intended to help identify children who are at increased risk of CVD and may benefit from the early screening and intervention of dyslipidemia. These guidelines provide a schematic approach that will help pediatricians make timely decisions regarding the screening and management of Korean children and adolescents with risks and conditions associated with accelerated atherosclerosis.
ACKNOWLEDGMENTS
The authors greatly appreciate the members of Committee on Dyslipidemia of Korean Pediatric and Adolescents and the Korean Society for Pediatric Endocrinology for their support in developing and publishing these guidelines. This guideline is being simultaneously published in Annals of Pediatric Endocrinology & Metabolism and Clinical and Experimental Pediatrics.
Fig.┬Ā1.
Algorithm for dyslipidemia treatment. FLP, fasting lipid profile; LDL-C, low-density lipoprotein cholesterol; CHILD 1, Cardiovascular Health Integrated Lifestyle Diet 1; CHILD 2-LDL, Cardiovascular Health Integrated Lifestyle Diet 2; non-HDL-C, nonŌĆōhigh-density lipoprotein cholesterol; TG, triglycerides; CVD, cardiovascular disease.
Table┬Ā1.
Definition of dyslipidemia in children and adolescents
| Variable | Acceptable | Borderline* | AbnormalŌĆĀ |
|---|---|---|---|
| Total cholesterol (mg/dL) | <170 | 170ŌĆō199 | Ōēź200 |
| LDL-C (mg/dL) | <110 | 110ŌĆō129 | Ōēź130 |
| NonŌĆōHDL-C (mg/dL) | <120 | 120ŌĆō144 | Ōēź145 |
| Triglyceride (mg/dL) | |||
| ŌĆā0ŌĆō9 years | <75 | 75ŌĆō99 | Ōēź100 |
| ŌĆā10ŌĆō19 years | <90 | 90ŌĆō129 | Ōēź130 |
| HDL-C (mg/dL) | >45 | 40ŌĆō45 | <40 |
LDL-C, low-density lipoprotein cholesterol; Non-HDL-C, nonŌĆōhigh-density lipoprotein cholesterol; HDL-C, high-density lipoprotein.
ŌĆĀ Abnormal values of total cholesterol and LDL-C represent the 95th percentile, except for HDL-C which represents the 10th percentile. [16]
Adapted from National Heart, Lung, and Blood Institute 2011. [20] Non-HDL-C=TCŌĆōHDL-C.
Table┬Ā2.
Risk factors of dyslipidemia
Table┬Ā3.
Screening for dyslipidemia
| Age | Recommendation |
|---|---|
| BirthŌĆō2 yr | No lipid screening |
| 2ŌĆō8 yr | No routine lipid screening |
| Measure fasting lipid profile if child has a family history of dyslipidemia, moderate or high-risk factors and condition* | |
| 9ŌĆō11 yr | Universal screening |
| Measure nonfasting nonŌĆōHDL-C | |
| Fasting lipid testing if non-HDL-CŌēź145 mg/dL* | |
| 12ŌĆō16 yr | No routine lipid screening |
| Measure fasting lipid profile if child has a family history of dyslipidemia, moderate or high-risk factors and condition* | |
| 17ŌĆō21 yr | Universal screening |
| Measure nonfasting non-HDL-C | |
| Fasting lipid testing if non-HDL-CŌēź145 mg/dL* |
Table┬Ā4.
Recommendations of CHILD 1, CHILD 2-LDL, CHILD 2-TG diets
Table┬Ā5.
Recommendations for pharmacological treatment of dyslipidemia
Table┬Ā6.
Major effects and dose of medications for dyslipidemia
| Type of medication | Major effects | Adverse effects | Common names | Daily dose | FDA approval in children |
|---|---|---|---|---|---|
| HMG-CoA reductase inhibitors | ŌåōLDL cholesterol &TG, ŌåæHDL cholesterol | ŌåæLiver transaminases ŌåæCreatine kinase, myopathy, rhabdomyolysis | Lovastatin (Mevacor) | 20ŌĆō80 mg | Approved |
| Simvastatin (Zocor) | 20ŌĆō80 mg | Approved | |||
| Pravastatin (Pravachol) | 20ŌĆō80 mg | Approved | |||
| Atorvastatin (Lipitor) | 5ŌĆō80 mg | Approved | |||
| Cholesterol absorption inhibitors | ŌåōLDL cholesterol | Myopathy, gastrointestinal upset | Ezetimibe | 10 mg | Not approved |
| Fibric acid derivatives | ŌåōTG, ŌåæHDL cholesterol | Dyspepsia, constipation, myositis, anemia | Gemfibrozil | 1,200 mg | Not approved |
| Fenofibrate | 48ŌĆō145 mg | Not approved | |||
| Clofibrate | 2 g* | Not approved | |||
| Omega-3-fish oil | ŌåōTG | Docosahexaenoic acid (DHA) | 2ŌĆō4 g (adults) | Not approved | |
| Nicotinic acid (extended release) | ŌåōTG & LDL cholesterol | Flushing, hepatic toxicity | 1,000ŌĆō2,250 mg* | ||
| Bile acid sequestrants | ŌåōLDL cholesterol, ŌåæTG | Limited to gastrointestinal tract; gas, bloating, constipation, cramps | Cholestyramine | 8ŌĆō16 g #2 | ** |
| Colestipol | 2.5ŌĆō20 g | ** | |||
| Colesevelam | 1.25ŌĆō4.375 g | ** |
References
1. The Global Burden of Disease 2004 Update [Internet]. Geneva (Switzerland), World Health Organization. 2008;[cited 2020 May 3]. Available from: https://www.who.int/healthinfo/global_burden_disease/GBD_report_2004update_full.pdf.
2. Korea National Statistical Office. Annual report on the cause of death statistics, 2010 [Internet]. Daejeon (Korea), Statistics Korea. [cited 2020 May 3]. Available from: http://kostat.go.kr/portal/eng/index.action.
3. Berenson GS, Srinivasan SR, Bao W, Newman WP 3rd, Tracy RE, Wattigney WA. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. The Bogalusa Heart Study. N Engl J Med 1998;338:1650ŌĆō6.


4. Stamler J, Daviglus ML, Garside DB, Dyer AR, Greenland P, Neaton JD. Relationship of baseline serum cholesterol levels in 3 large cohorts of younger men to long-term coronary, cardiovascular, and all-cause mortality and to longevity. JAMA 2000;284:311ŌĆō8.


5. Sone H, Tanaka S, Tanaka S, Iimuro S, Oida K, Yamasaki Y, et al. Serum level of triglycerides is a potent risk factor comparable to LDL cholesterol for coronary heart disease in Japanese patients with type 2 diabetes: subanalysis of the Japan Diabetes Complications Study (JDCS). J Clin Endocrinol Metab 2011;96:3448ŌĆō56.


6. Goldbourt U, Yaari S, Medalie JH. Isolated low HDL cholesterol as a risk factor for coronary heart disease mortality. A 21-year follow-up of 8000 men. Arterioscler Thromb Vasc Biol 1997;17:107ŌĆō13.


7. Jee SH, Jang Y, Oh DJ, Oh BH, Lee SH, Park SW, et al. A coronary heart disease prediction model: the Korean Heart Study. BMJ Open 2014;4:e005025.



8. McGill HC Jr, McMahan CA, Zieske AW, Tracy RE, Malcom GT, Strong JP. Effects of nonlipid risk factors on atherosclerosis in youth with a favorable lipoprotein profile. Circulation 2001;103:1546ŌĆō50.


9. Ko M, Kim MT, Nam JJ. Assessing risk factors of coronary heart disease and its risk prediction among Korean adults: the 2001 Korea National Health and Nutrition Examination Survey. Int J Cardiol 2006;110:184ŌĆō90.


10. Kim S, Moon S, Popkin BM. The nutrition transition in South Korea. Am J Clin Nutr 2000;71:44ŌĆō53.



11. Lauer RM, Lee J, Clarke WR. Factors affecting the relationship between childhood and adult cholesterol levels: the Muscatine Study. Pediatrics 1988;82:309ŌĆō18.

12. Kavey RE, Daniels SR, Lauer RM, Atkins DL, Hayman LL, Taubert K, et al. American Heart Association guidelines for primary prevention of atherosclerotic cardiovascular disease beginning in childhood. Circulation 2003;107:1562ŌĆō6.


13. Raitakari OT, Juonala M, Kahonen M, Taittonen L, Laitinen T, Maki-Torkko N, et al. Cardiovascular risk factors in childhood and carotid artery intima-media thickness in adulthood: the Cardiovascular Risk in Young Finns Study. JAMA 2003;290:2277ŌĆō83.



14. McGill HC Jr, McMahan CA, Malcom GT, Oalmann MC, Strong JP. Effects of serum lipoproteins and smoking on atherosclerosis in young men and women. The PDAY Research Group. Pathobiological Determinants of Atherosclerosis in Youth. Atheroscler Thromb Vasc Biol 1997;17:95ŌĆō106.

15. Kim SH, Ahn BC, Joung H, Park MJ. Lipid profiles and prevalence of dyslipidemia in Korean adolescents. Endocrinol Metab 2012;27:208ŌĆō16.

16. Yang S, Hwang JS, Park HK, Lee HS, Kim HS, Kim EY, et al. Serum lipid concentrations, prevalence of dyslipidemia, and percentage eligible for pharmacological treatment of Korean children and adolescents; data from the Korea National Health and Nutrition Examination Survey IV (2007-2009). PLoS One 2012;7:e49253.



17. Hickman TB, Briefel RR, Carroll MD, Rifkind BM, Cleeman JI, Maurer KR, et al. Distributions and trends of serum lipid levels among United States children and adolescents ages 4ŌĆō19 years: data from the Third National Health and Nutrition Examination Survey. Prev Med 1998;27:879ŌĆō90.


18. Ford ES, Li C, Zhao G, Mokdad AH. Concentrations of low-density lipoprotein cholesterol and total cholesterol among children and adolescents in the United States. Circulation 2009;119:1108ŌĆō15.


19. Shim YS, Baek JW, Kang MJ, Oh YJ, Yang S, Hwang IT. R eference values for the triglyceride to high-density lipoprotein cholesterol ratio and non-high-density lipoprotein cholesterol in Korean Children and Adolescents: the Korean National Health and Nutrition Examination Surveys 2007-2013. J Atheroscler Thromb 2016;23:1334ŌĆō44.



20. Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents; National Heart, Lung, and Blood Institute. Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: summary report. Pediatrics 2011;128(Suppl 5):S213ŌĆō56.



21. Havel PJ. Dietary fructose: implications for dysregulation of energy homeostasis and lipid/carbohydrate metabolism. Nutr Rev 2005;63:133ŌĆō57.



22. Lichtenstein AH, Appel LJ, Brands M, Carnethon M, Daniels S, Franch HA, et al. Summary of American Heart Association Diet and Lifestyle Recommendation revision 2006. Arterioscler Thromb Vasc Biol 2006;26:2186ŌĆō91.


23. US Preventive Services Task Force. Screening for lipid disorders in children: US Preventive Services Task Force recommendation statement. Pediatrics 2007;120:e215ŌĆō9.


24. Haney EM, Huffman LH, Bougatsos C, Freeman M, Fu R, Steiner RD, et al. Screening for lipid disorders in children and adolescents [Internet]. Rockville, Agency for Healthcare Research and Quality. 2007;[cited 2020 May 3]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK33480/.
25. Slack J. Risks of ischaemic heart-disease in familial hyperlipoproteinaemic states. Lancet 1969;2:1380ŌĆō2.


26. Srinivasan SR, Frontini MG, Xu J, Berenson GS. Utility of childhood non-high-density lipoprotein cholesterol levels in predicting adult dyslipidemia and other cardiovascular risks: the Bogalusa Heart Study. Pediatrics 2006;118:201ŌĆō6.


27. Cu i Y, Blu ment ha l R S, Fl aw s JA, Whiteman M K, Langenberg P, Bachorik PS, Bush TL. Non-high-density lipoprotein cholesterol level as a predictor of cardiovascular disease mortality. Arch Intern Med 2001;161:1413ŌĆō9.


28. Frontini MG, Srinivasan SR, Xu JH, Tang R, Bond MG, Berenson G. Utility of non-high-density lipoprotein cho┬¼lesterol versus other lipoprotein measures in detecting subclinical atherosclerosis in young adults (The Bogalusa Heart Study). Am J Cardiol 2007;100:64ŌĆō8.


29. Hong HR, Kim SU, Kang HS. Physical activity and metabolic syndrome in Korean children. Int J Sports Med 2009;30:677ŌĆō83.



30. Shin KO, Oh SY, Park HS. Empirically derived major dietary patterns and their associations with overweight in Korean preschool children. Br J Nutr 2007;98:416ŌĆō21.


31. US department of agriculture; US Department of Health and Human Services. Dietary Guidelines for Americans, 2010. 7th ed. Washington, DC: US Government Printing Office. 2011.
32. Becque MD, Katch VL, Rocchini AP, Marks CR, Moorehead C. Coronary risk incidence of obese adolescents: reduction by exercise plus diet intervention. Pediatrics 1988;81:605ŌĆō12.



33. McCrindle BW, American Heart Association Writing Group; Urbina EM, Dennison BA, Jacobson MS, Steinberger J, et al. Summary of the American Heart Association's scientific statement on drug therapy of high-risk lipid abnormalities in children and adolescents. Arterioscler Thromb Vasc Biol 2007;27:982ŌĆō5.


34. McCrindle BW, Urbina EM, Dennison BA, Jacobson MS, Steinberger J, Rocchini AP, et al. Drug therapy of highrisk lipid abnormalities in children and adolescents: a scientific statement from the American Heart Association Atherosclerosis, Hypertension, and Obesity in Youth Committee, Council of Cardiovascular Disease in the Young, with the Council on Cardiovascular Nursing. Circulation 2007;115:1948ŌĆō67.


36. Manlhiot C, Larsson P, Gurofsky RC, Smith RW, Fillingham C, Clarizia NA, et al. Spectrum and management of hypertriglyceridemia among children in clinical practice. Pediatrics 2009;123:458ŌĆō65.


37. Berglund L, Brunzell JD, Goldberg AC, Goldberg IJ, Sacks F, Murad MH, et al. Evaluation and treatment of hypertriglyceridemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2012;97:2969ŌĆō89.




38. Wiegman A, Hutten BA, de Groot E, Rodenburg J, Bakker HD, Buller HR, et al. Efficacy and safety of statin therapy in children with familial hypercholesterolemia: a randomized controlled trial. JAMA 2004;292:331ŌĆō7.


39. McCrindle BW, Ose L, Marais AD. Efficacy and safety of atorvastatin in children and adolescents with familial hypercholesterolemia or severe hyperlipidemia: a multicenter, randomized, placebo-controlled trial. J Pediatr 2003;143:74ŌĆō80.


40. de Jongh S, Ose L, Szamosi T, Gagne C, Lambert M, Scott R, et al. Efficacy and safety of statin therapy in children with familial hypercholesterolemia: a randomized, doubleblind, placebo-controlled trial with simvastatin. Circulation 2002;106:2231ŌĆō7.


41. Avis HJ, Vissers MN, Stein EA, Wijburg FA, Trip MD, Kastelein JJ, et al. A systematic review and metaanalysis of statin therapy in children with familial hypercholesterolemia. Arterioscler Thromb Vasc Biol 2007;27:1803ŌĆō10.









