 |
 |
- Search
| Ann Pediatr Endocrinol Metab > Volume 20(2); 2015 > Article |
|
Abstract
We report a 13-year-old girl with Graves disease, who showed an increased level of serum creatine kinase (CK) accompanied by myalgia after methimazole (MMI) treatment. This patient developed muscular pain two weeks after MMI administration, along with increased CK levels. The level of thyroid hormone was within the normal range when she showed increased CK levels. After the MMI dose was decreased and levo-thyroxine was added, serum CK levels decreased to normal and the myalgia improved. The pathophysiologic mechanism of this effect has not yet been elucidated. An acute relatively hypothyroid state occurs secondary to antithyroid drug (ATD) administration in chronic hyperthyroidism, which may cause changes in the CK levels. In this report, we present a rare pediatric case, along with a literature review of similar cases. In the initial state of MMI treatment, myalgia should be detected and when it occurs, CK levels should be measured. The clinical strategy of monitoring CK levels with the aim of normalizing thyroid hormones is helpful in case of the development of adverse reactions, such as myalgia, during ATD treatment for Graves disease in children.
Graves disease is the most common disorder in children and adolescents under treatment for hyperthyroidism. It occurs in about 0.02% of children, and develops more frequently in females than males for both adults and children1,2). The treatment options include antithyroid drug, radioactive iodine therapy, and surgery. Oral medication is the firstline treatment of choice, rather than radioactive iodine therapy or surgery. Methimazole (MMI) is the initial drug of choice for the treatment of hyperthyroidism in children1,3). MMI's common but relatively-mild adverse reactions, such as pruritus and skin rash, may be spontaneously resolved even if the drug is continuously administered. Adverse reactions, such as agranulocytosis, acute toxic hepatitis or vasculitis are not common, but serious, so if such adverse reactions occur, MMI must be discontinued immediately and alternative treatments administered4,5).
A few patients with Graves disease were reported suffering from myalgia with an elevation of serum creatine kinase (CK) levels after MMI treatment. This adverse reaction is relatively rare and a child with such an adverse reaction has never been reported in Korea. We report a rare case of Graves disease in a child who developed myalgia accompanied by a CK increase after initiation of MMI administration, whose signs and symptoms improved after reduction of the MMI dosage and addition of levo-thyroxine (L-thyroxine). Also, we review the previous cases of seven patients with an elevation of serum CK resulting from MMI treatment of Graves disease.
A 13-year-old girl born at a gestational age of 38 weeks and a body weight of 2.6 kg was referred to the Pediatric Endocrinology Clinic at the University of Michigan (Health System) for evaluation of exophthalmos and an enlarged thyroid gland. She had a complaint of fatigue. She had no past medical or family history. On initial examination, her heart rate and blood pressure were 86/min and 113/58 mmHg, respectively, while her height and body weight were 158 cm (0.49 standard deviation score [SDS]) and 67.8 kg (2.5 SDS), respectively. She experienced intermittent palpitations and sweating accompanied by weight loss. Her laboratory results showed that her serum TSH, free T3, and free T4 were 0.01 ┬ĄIU/mL (reference range [RR], 0.88-4.65 ┬ĄIU/mL), 0.02 pmol/L (RR, 2.0-7.0 pmol/L), and 4.7 ng/dL (RR, 0.96-1.52 ng/dL), respectively6,7,8). She was diagnosed with a grade I goiter in accordance with World Health Organization's classification 9). Ultrasonography of her neck showed a mild swelling of the thyroid gland. Based on her clinical symptoms and laboratory findings, the patient was diagnosed as having Graves disease. The patient started to take 7.5 mg MMI twice a day (0.25 mg/kg/day) and after that, her clinical symptoms related to hyperthyroidism, such as palpitations and sweating with weight loss, showed improvement. However, muscular pain developed two weeks after starting medication with MMI at this dose. The sharp muscle pain occurred in her shoulder, sides, calf, and posterior thigh when she tried to change positions. The pain episodes occurred about 5 times per week, with each episode usually lasting for about ten seconds. Because symptoms related to hyperthyroidism showed improvement, her myalgia was observed without any further action. At two months after starting MMI treatment, the patient's myalgia became aggravated; therefore, her CK levels were evaluated. Her CK level had dramatically increased above the normal range to 1,784 IU/L (RR, 45-257 IU/L)7). Laboratory tests to rule out common causes of elevated CK levels were conducted, including rheumatoid factor, erythrocyte sedimentation rate, C-reactive protein, lactate dehydrogenase, calcium, phosphate, antinuclear antibody, and CK 2 isoenzyme, which were all within normal ranges. A follow-up laboratory result showed a further increase of the serum CK levels up to 3,339 IU/L. Because she consistently remained in an euthyroid state, the MMI dosage of 15 mg/day was reduced to 10 mg/day.
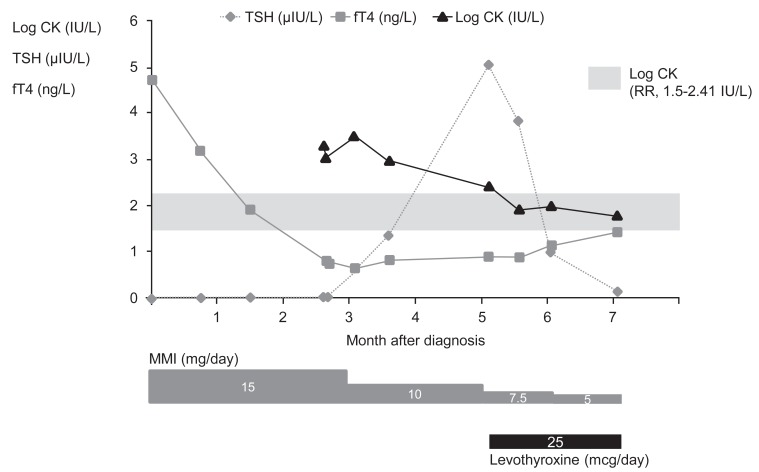
She was referred to the Samsung Medical Center due to the close proximity of this hospital to her hometown and she underwent a further evaluation two months after the reduction of the MMI dosage. On examination, her height was 159.6 cm (0.32 SDS) and her body weight was 64.5 kg (1.73 SDS). On physical examination, she had a goiter (grade I) but did not exhibit symptoms of hyperthyroidism. A laboratory test showed that levels of serum TSH, total T3 and free T4 levels were 5.038 ┬ĄIU/mL, 1.63 nmol/L, and 0.91 ng/dL, respectively. Serum thyroglobulin, antithyroid microsomal, and TSH-receptor antibody levels were 48.0 IU/mL (RRŌēż20 IU/mL), 209.5 IU/mL, and 10.9 IU/L (RRŌēż1.75 IU/L), respectively. The serum CK level was 265 IU/L, which was still elevated even after a dose reduction of MMI for two months. Because CK levels were still elevated and myalgia still remained, the dosage of MMI was further reduced to 7.5 mg daily, and L-thyroxine (25 ┬Ąg) was added. After two weeks, the serum CK level decreased to within the normal range (81 IU/L), and the myalgia finally disappeared. At that time, the levels of serum TSH, total T3, and free T4 were 3.832 ┬ĄIU/mL, 1.19 nmol/L, and 0.88 ng/dL, respectively. Approximately nine months after diagnosis, this patient was placed on a regimen of MMI (5 mg daily) with L-thyroxine (25 ┬Ąg) and she exhibited euthyroid status without myalgia (Fig. 1).
Myalgia with increased serum CK levels usually make clinicians suspect collagen-vascular disease, viral-induced myositis, myocardial damage, or neuromuscular disorders, such as Duchenne dystrophy. Serum CK is usually normal in thyrotoxicosis and high in hypothyroidism10). However, there are several case reports of elevations in serum CK levels during medical treatment for Graves disease, even in the absence of the hypothyroid state11,12,13). Therefore, when treating Graves disease, measurement of serum CK levels is needed when the patient shows symptoms of myalgia.
Our patient developed myalgia shortly after two weeks of MMI treatment and thyroid function returned to a euthyroid state after a treatment period of six weeks. CK level was increased (1,784 IU/L) at 2 months after initiation of MMI. Because the CK level was still increased and the patient's symptoms of myalgia remained, the dosage of MMI was further reduced and L-thyroxine was added to the treatment regimen. Following this regimen adjustment, the CK level decreased to within the normal range and the myalgia disappeared.
We reviewed the previous seven cases of Graves disease that were associated with an elevation of CK levels (Table 1). The commonly-shared aspect of these cases was that there was an onset of myalgia with a concurrent rise of the CK level within 1 to 2 months of initiation of MMI treatment. At the time of occurrence of myalgia, free T4 levels declined to within the normal range. Reduction of the MMI dosage and the addition of L-thyroxine resulted in CK normalization and symptomatic improvement without any further adverse effects. The CK level began to decrease after adding L-thyroxine administration in 6 out of the 7 cases. Mizuno et al.11) reported in one patient that the CK level decreased to within the normal range by a reduction of MMI only (30 to 20 mg/day) and discontinuation of a beta-blocker without the addition of L-thyroxine. In the case of Ito et al.13), the clinician replaced MMI with propylthiouracil (PTU) but the serum CK level was still elevated and hyperthyroidism persisted. After PTU was changed to MMI along with L-thyroxine, CK levels decreased to normal with an improvement of the hyperthyroid state and a relief of myalgia. The hypothyroid state was not noted during MMI management in the study cases reviewed.
The exact cause of this phenomenon remains unclear. The mechanisms by which antithyroid agents cause an elevation of serum CK in Graves disease include (1) a direct effect of ATD on muscles, (2) the result of a concentration imbalance due to an interference in the production of thyroid hormone11) and (3) effects mediated by the inhibition of the production of thyroid hormones13,14). In our case, myalgia was accompanied by a CK rise during MMI treatment without a hypothyroid state followed by a normalized CK level with symptomatic improvement, which may occur after a dosage reduction and supplementation of a thyroid hormone. Therefore, we can postulate that a rapid decrease of thyroid hormone leads to a relatively hypothyroid state, which can cause transient high serum CK concentrations. For this reason, we did not have to conduct an unnecessary invasive work-up such as muscle biopsy to exclude other neuromuscular diseases15).
In conclusion, we describe the elevation of serum CK during MMI treatment in a case of pediatric Graves disease. Although rare, clinicians should be aware of this potential side effect when initiating treatment for Graves disease in children. Therefore, myalgia must be monitored closely in the initial state of MMI treatment. CK measurement may serve as a good tool when patients present with myalgia.
References
1. Kaguelidou F, Carel JC, Leger J. Graves' disease in childhood: advances in management with antithyroid drug therapy. Horm Res 2009;71:310ŌĆō317. PMID: 19506387.


2. Rivkees SA. Pediatric Graves' disease: management in the post-propylthiouracil Era. Int J Pediatr Endocrinol 2014;2014:10. PMID: 25089127.



4. Marino M, Latrofa F, Menconi F, Chiovato L, Vitti P. An update on the medical treatment of Graves' hyperthyroidism. J Endocrinol Invest 2014;9 04 [Epub]. PMID: 10.1007/s40618-014-0136-z.

5. Nihei H, Tada H, Naruse Y, Izawa M, Kato M, Okuno H, et al. Polyarthritis caused by methimazole in two Japanese patients with graves' disease. J Clin Res Pediatr Endocrinol 2013;5:270ŌĆō272. PMID: 24379039.




6. Chaler EA, Fiorenzano R, Chilelli C, Llinares V, Areny G, Herzovich V, et al. Age-specific thyroid hormone and thyrotropin reference intervals for a pediatric and adolescent population. Clin Chem Lab Med 2012;50:885ŌĆō890. PMID: 22628332.



7. Clifford SM, Bunker AM, Jacobsen JR, Roberts WL. Age and gender specific pediatric reference intervals for aldolase, amylase, ceruloplasmin, creatine kinase, pancreatic amylase, prealbumin, and uric acid. Clin Chim Acta 2011;412:788ŌĆō790. PMID: 21238443.


8. Zurakowski D, Di Canzio J, Majzoub JA. Pediatric reference intervals for serum thyroxine, triiodothyronine, thyrotropin, and free thyroxine. Clin Chem 1999;45:1087ŌĆō1091. PMID: 10388488.


9. Kalra S, Khandelwal SK, Goyal A. Clinical scoring scales in thyroidology: A compendium. Indian J Endocrinol Metab 2011;15(Suppl 2):S89ŌĆōS94. PMID: 21966660.



10. Scott KR, Simmons Z, Boyer PJ. Hypothyroid myopathy with a strikingly elevated serum creatine kinase level. Muscle Nerve 2002;26:141ŌĆō144. PMID: 12115960.


11. Mizuno H, Sugiyama Y, Nishi Y, Ueda N, Ohro Y, Togari H. Elevation of serum creatine kinase in response to medical treatment of Graves' disease in children. Acta Paediatr 2006;95:243ŌĆō245. PMID: 16449033.


12. Suzuki S, Ichikawa K, Nagai M, Mikoshiba M, Mori J, Kaneko A, et al. Elevation of serum creatine kinase during treatment with antithyroid drugs in patients with hyperthyroidism due to Graves disease. A novel side effect of antithyroid drugs. Arch Intern Med 1997;157:693ŌĆō696. PMID: 9080924.


13. Ito T, Katahira M, Hanakita M, Suzuki M. A case of elevation of serum creatine kinase with antithyroid medications for Graves disease. J Endocrinol Metab 2012;2:244ŌĆō247.

14. Iwaku K, Noh JY, Minagawa A, Kosuga Y, Suzuki M, Sekiya K, et al. Determination of pediatric reference levels of FT3, FT4 and TSH measured with ECLusys kits. Endocr J 2013;60:799ŌĆō804. PMID: 23563672.


15. Shaibani A, Jabari D, Jabbour M, Arif C, Lee M, Rahbar MH. Diagnostic outcome of muscle biopsy. Muscle Nerve 2015;51:662ŌĆō668. PMID: 25187298.


Fig.┬Ā1
Serum concentrations of both TSH and fT4 with the common logarithms of the CK level (log CK). Logarithmic scales were used to graph TSH, fT4, and CK levels. Horizontal axis: the extended month after the diagnosis of Graves disease. TSH, thyroid-stimulating hormone; fT4, free thyroxine; CK, creatine kinase; RR, reference range; MMI, methimazole.
Table┬Ā1.
Comparison of cases increased serum CK levels during methimazole treatment in Graves disease between previous studies and our case
| Variable |
Mizuno et al. [11] |
Suzuki et al. [12] |
Ito et al. [13] | Our case | ||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 1 | 2 | 3 | 4 | |||
| Age at diagnosis (yr) | 12 | 14 | 44 | 27 | 26 | 20 | 43 | 13 |
| Sex | F | F | M | F | F | F | F | F |
| Initial TSH (┬ĄIU/mL) | <0.1 | 0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.01 | 0.01 |
| Initial free T4 (ng/dL) | 15.7 | 8.33 | NR | NR | NR | NR | 3.64 | 4.7 |
| Initial treatment | MMI (30 mg/day)+╬▓-blocker | MMI (30 mg/day) | MMI (dose NR) | MMI (30 mg/day) | MMI (30 mg/day) | MMI (30 mg/day) | MMI (15 mg/day)+╬▓-blocker | MMI (15 mg/day) |
| Development of myalgia after medication (wk) | 4 | 4 | 2 | None | 4 | 4 | 8 | 2 |
| Initial CK level check after first treatment (wk) | 4 | 4 | 0 | 8 | 4 | 4 | 16 | 8 |
| Maximum increase in CK level (IU/L) | 2,651 | 11,630 | 1,600 | 520 | 270 | 1,900 | 4,791 | 3,339 |
| TSH (┬ĄIU/mL)a) | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | 0.05 | 0.59 |
| Free T4 (ng/dL)a) | 0.2 | NR | NR | NR | NR | NR | 0.82 | 0.66 |
| T4 (┬Ąg/mL)a) | NR | 9 | 7.02 | 7.55 | 7.55 | 13.16 | NR | Not done |
| Management | Reduction of MMI dose (20 mg/day), discontinuation of ╬▓-blocker | Change MMI to PTU, L-thyroxine addition | Reduction of MMI dose, L-thyroxine addition | Reduction of MMI dose, L-thyroxine addition | Reduction of MMI dose, L-thyroxine addition | L-thyroxine addition (50 ╬╝mg) | Discontinuation of MMI, Maintenance ╬▓-blocker, KI addition | Reduction of MMI dose, L-thyroxine addition |
Serum TSH (reference range [RR], 0.88ŌĆō4.65 ╬╝IU/mL); free T4 (RR, 0.96ŌĆō1.52 ng/dL); T4 (RR, 5.5ŌĆō12.5 ╬╝g/mL).
CK, creatine kinase; TSH, thyroid-stimulating hormone; free T4, free thyroxine; T4, thyroxine total; NR, not reported; MMI, methimazole; PTU, propylthiouracil ; L-thyroxine, levo-thyroxine; KI, potassium iodide.
- TOOLS
-
METRICS

-
- 8 Crossref
- Scopus
- 9,328 View
- 148 Download
- Related articles in APEM





